Abstract
This assignment analyzes a patient’s blood Total Bilirubin and ELISA-based CA 19-9 antigen biochemical testing. The patient’s ELISA-based CA 19-9 antigen test was positive. The patient’s Total Bilirubin test showed an absorbance value of 0.05, suggesting a 12 μmol/l concentration within the normal range of 1.7 to 20.5. The total bilirubin calibration curve exhibited a linear correlation of 0.996, demonstrating a significant positive association between absorbance and concentration. This investigation found the CA 19-9 antigen in the patient’s serum, indicating pancreatic cancer. Another test must confirm pancreatic cancer. The patient’s serum Total Bilirubin is normal. Several studies have shown that the ELISA-based CA 19-9 antigen test can diagnose and monitor pancreatic cancer. The Total Bilirubin test is a routine liver function test typically used alongside other diagnostic tests to diagnose liver disease. The short sample size and lack of a pancreatic cancer confirmatory test restrict this investigation. Future research should include more diagnostic tests for liver and pancreatic function and a bigger sample size.
In conclusion, CA 19-9 antigen in the patient’s serum may indicate pancreatic cancer. More tests are required to confirm the diagnosis. Total Bilirubin values showed no liver impairment.
Introduction
Total bilirubin quantifies blood bilirubin. Red blood cell breakdown produces Bilirubin, and liver processing excretes it; hence Hyperbilirubinemia may result from liver dysfunction (Bhattacharya, Tyagi & Singh, 2020). Jaundice caused by high bilirubin levels, liver disease, bile duct blockage, and other liver disorders are diagnosed using total bilirubin levels.
A tumor marker test measures blood CA 19-9 antigen. Pancreatic, ovarian, and colorectal cancer cells make CA 19-9. The CA 19-9 test is routinely used alongside imaging investigations to identify or track cancer. ELISA (Enzyme-linked immunosorbent assay) is a standard laboratory method for measuring blood protein and antigen levels (Kaya & Korkmaz, 2017). The serum sample is combined with a CA 19-9 antigen-binding antibody in an ELISA-based CA 19-9 antigen assay. The blood sample’s CA 19-9 antigen will bind to the antibody, generating an antigen-antibody combination (Li et al., 2018). Colorimetric or fluorescence signals detect the complex. In conclusion, total Bilirubin is utilized to detect liver function and bile duct blockage, whereas the ELISA-based CA 19-9 antigen test helps identify and track cancer. Both procedures measure blood biomolecules and offer health information.
A 53-year-old lady presents to the James Cook University Hospital in Middlesbrough with acute burning and squeezing epigastric pain without nausea, vomiting, or diarrhea. The patient experienced repeated hospitalizations for comparable discomfort. The patient has hypertriglyceridemia, chronic renal disease, and diabetes. Her abdomen is fragile with epigastric discomfort, guarding, and reduced bowel noises.
On admission, serum lipase activity was 1506 U/L, above standard limits. Triglycerides were 1606 mg/dL, much over the standard range. HDL was 14 mg/dL, and total cholesterol was 205 mg/dL. The reference index was met by creatinine at 1.1 mg/dL and blood urea nitrogen at 14 mg/dL (5.0 mmol/L). A computed Topography scan was ordered for structural problems.
The patient’s history and biochemical data indicate acute pancreatitis with hypertriglyceridemia, chronic renal disease, and diabetes. Acute pancreatitis causes epigastric discomfort, tenderness, and guarding. The elevated blood triglycerides imply hypertriglyceridemia, a risk factor for acute pancreatitis. Chronic renal disease and diabetes may have caused pancreatitis. A positive ELISA-based CA 19-9 antigen test for pancreatic cancer may suggest acute pancreatitis, not malignancy. Thus, the positive CA 19-9 test result should not be considered a conclusive pancreatic cancer diagnosis in this patient without a computed topography report. The patient’s Total Bilirubin test indicated 12 μmol/l, within the normal range of 1.7 to 20.5. The patient’s liver function seems unaffected by pancreatitis (Singh et al., 2017). Acute pancreatitis is inflammation of the pancreas, a gland below the stomach that generates enzymes and hormones for digestion and glucose metabolism. Acute pancreatitis causes tissue injury and inflammation by activating pancreatic enzymes and releasing those (Singh et al., 2017). Acute pancreatitis is caused by pancreatic digestive enzymes self-digesting tissue. Until they reach the small intestine, pancreatic enzymes remain inert. Acute pancreatitis activates these enzymes inside the pancreas, damaging pancreatic cells and adjacent organs.
Several methods may activate pancreatic enzymes, including:
- Gallstones: Blocking the bile duct, which drains into the pancreatic duct, causes pancreatic enzymes to accumulate.
- Alcohol consumption: Chronic alcohol use might induce pancreatic enzyme buildup and duct blockage.
- Trauma: Abdominal trauma damages the pancreas, activating enzymes.
- Mumps and viral hepatitis may inflame the pancreas.
Once triggered, pancreatic enzymes tear down pancreatic tissue and adjacent tissues, and inflammatory mediators like cytokines promote pancreatic inflammation and edema. Pancreatic necrosis may result from severe inflammation. Acute pancreatitis causes stomach discomfort, nausea, vomiting, and fever. Blood tests reveal increased pancreatic enzymes such as amylase and lipase. CT scans can diagnose and assess inflammation. Acute pancreatitis treatment includes pain control, hydration, and nourishment. Hospitalization and pancreatic tissue removal may be needed in extreme situations. This laboratory report analyzes the patient’s serum sample for Total Bilirubin and ELISA-based CA 19-9 antigen to predict and discuss the disease diagnosis and prognosis based on history and biochemical measurements.
Methods
In this Experiment, the serum sample of the 53-year-old woman was presented to the laboratory, and a Total Bilirubin test, as well as an ELISA-based CA 19-9 antigen test, was carried out through different methods to predict and discuss the disease diagnosis and prognosis for this patient according to the biochemical measurements.
ELISA-based CA 19-9 antigen test
ELISA-based immunoassays detect CA 19-9 antigen in liquid samples. Pancreatic cancer patients often have the carbohydrate antigen CA 19-9 in their blood. The test uses CA 19-9-binding antibodies. Antibodies are coated on a plastic well. After adding a liquid sample to the well, the antibodies will attach to any CA 19-9 antigen. After antigen-antibody binding, the well is rinsed to eliminate unbound components. The well receives a secondary antibody. This enzyme-coupled secondary antibody targets CA 19-9. CA 19-9 antigen in the sample will bind to the secondary antibody and create a signal that may be identified by measuring enzyme activity.
A signal is proportional to CA 19-9 antigen in the sample. CA 19-9 antigen may be detected in an unknown sample by comparing its signal to established positive and negative controls. The extremely sensitive and specific ELISA-based CA 19-9 antigen test is used to diagnose pancreatic cancer and other cancers that generate this antigen. This ELISA experiment detects CA 19-9 antigen in an unknown sample. A 12-well strip is labeled with positive controls, negative controls, and the unknown sample for the ELISA. Positive and negative controls guarantee test accuracy. 50 μl of each of the positive, negative, and unknown controls are added to their designated wells. The strip is left to bind proteins to plastic wells for 5 minutes.
Washing removes unattached proteins from the wells. Each well receives a wash buffer once the microplate strip is inverted on paper towels. The strip is rinsed twice to remove unbound primary antibodies from wells. After adding 50 μl of the primary antibody to all 12 wells, the strip is left for 5 minutes to enable the antibodies to bind. Rewashing the wells removes the unbound primary antibody.
The secondary antibody is added to the wells, and the strip is allowed for 5 minutes to bind to its targets. Three times washing removes unbound secondary antibodies from wells. The strip is left for 5 minutes after adding enzyme substrate to all 12 wells. Results are noted. Comparing the unknown sample’s findings with the positive and negative controls determines its CA 19-9 antigen status. All samples are conducted in duplicate for accuracy and repeatability.
Total Bilirubin
A typical blood test for Bilirubin is the Total Bilirubin test. When red blood cells die, they release Bilirubin. It is liver-processed and bile-excreted. High blood bilirubin levels may suggest liver disease or red blood cell disintegration. The Total Bilirubin test uses a blood sample and a reagent to color bilirubin. Bilirubin levels directly affect color intensity. Spectrophotometers measure color in micromoles per liter. Biochemical scientists must comprehend its concepts and procedures to effectively and efficiently execute the Total Bilirubin test, analyze the findings, and troubleshoot errors. Bilirubin’s involvement in liver function and other medical disorders must also be understood.
The total bilirubin test requires bilirubin reagent, blanking reagent, DI water, calibration standard, serum quality control, spectrophotometer cuvettes, pipette tips, graph paper, and Microsoft Excel. The spectrophotometer warms up for 15 minutes. In exercise 1, a 2-point calibration curve is created by pipetting 380 μl of DI water into one spectrophotometer cuvette and 380 μl calibrator standard into the other, then adding 2 ml of reagent and mixing. After 10 minutes at room temperature, the absorbance of each sample is measured at 540nm using DI as a blank. The calibration curve is produced on graph paper with a concentration on the x-axis and absorbance on the y-axis.
Exercise 2 tests the calibration curve using the medium Bilirubin QC sample. Exercise 1 is repeated, but DI water and calibrator replace the QC sample. Exercise 3 tests an unknown patient sample for Total Bilirubin using the newly established standard curve. Pipet the patient sample into a spectrophotometer cuvette, add reagent, incubate, and measure absorbance at 540nm. The calibration curve from exercise 1 calculates total bilirubin concentration from absorbance.
Results
ELISA-based CA 19-9 antigen test
ELISA detects CA 19-9 antigen in samples. This antibody-based ELISA detects antigens. If the wells change color, this test is affirmative or negative. Antibodies attach to CA 19-9 antigens and modify the wells’ color. The intensity of the blue or purple color shift indicates the sample’s antigen content. Lighter colors indicate lower antigen concentrations, whereas darker colors suggest greater concentrations.
If the sample does not contain CA 19-9 antigen, the wells will not change color, and the test will be negative. Negative results imply no antigen in the sample. The ELISA-based CA 19-9 antigen test only detects the presence or absence of the antigen in the sample. It does not indicate antigen concentration (Wang & Yang, 2019). However, color shift intensity may indicate antigen concentration. ELISA-based CA 19-9 antigen testing is reliable and commonly used. It helps detect pancreatic, colorectal, and ovarian cancer. The case patient’s blood sample tested positive for CA 19-9 antigen by ELISA. Blue wells showed a good response, whereas no color change indicated an adverse reaction. The Experiment’s twelve wells showed that the carbohydrate antigen (CA) 19-9 test yielded a cheerful blue color.
ELISA-based CA 19-9 antigen test Results

Figure 1: ELISA-based CA 19-9 antigen test Results
Total Bilirubin
For the Total Bilirubin test, a calibration curve was constructed using two calibration points, DI water and a calibrator with a concentration of 56 µmol/l. The absorbance results for the QC sample at Level 2 was recorded at 0.10, which was used to determine the concentration of Bilirubin in the QC sample using the calibration curve. The measured QC concentration was within the acceptable range of 25-50 µmol/l, indicating that the test was accurate and reliable. Absorbance results recorded from the 2-point calibration practical as represented in the table below
| 2-point calibration curve absorbance results | |||
| Calibration point | Material | Concentration | Absorbance |
| 1st | DI water | 0 µmol/l | 0.02 |
| 2nd | Calibrator | 56µmol/l | 0.16 |
Table 1: Absorbance results recorded from the 2-point calibration practical
A calibration curve was then obtained as represented in Figure 2 below. An absorbance calibration curve is a graphical representation of the relationship between the concentration of a substance and the amount of light absorbed by that substance. In other words, it is a plot of the absorbance values (y-axis) against the known concentrations of a sample (x-axis). To create an absorbance calibration curve, a set of known standards with varying concentrations of the substance of interest are prepared, and their absorbance values are measured using a spectrophotometer. The data obtained is then plotted as a calibration curve. The curve typically shows a linear relationship between the concentration and absorbance values. The absorbance of the unknown sample is then measured, and its concentration is calculated using the calibration curve equation. The calibration curve is essential for the accurate determination of the concentration of a substance in an unknown sample. The curve allows the determination of the concentration of unknown samples by interpolating the absorbance value on the curve. The calibration curve also helps to validate the linearity and accuracy of the measurement instrument and method.
Absorbance calibration Curve
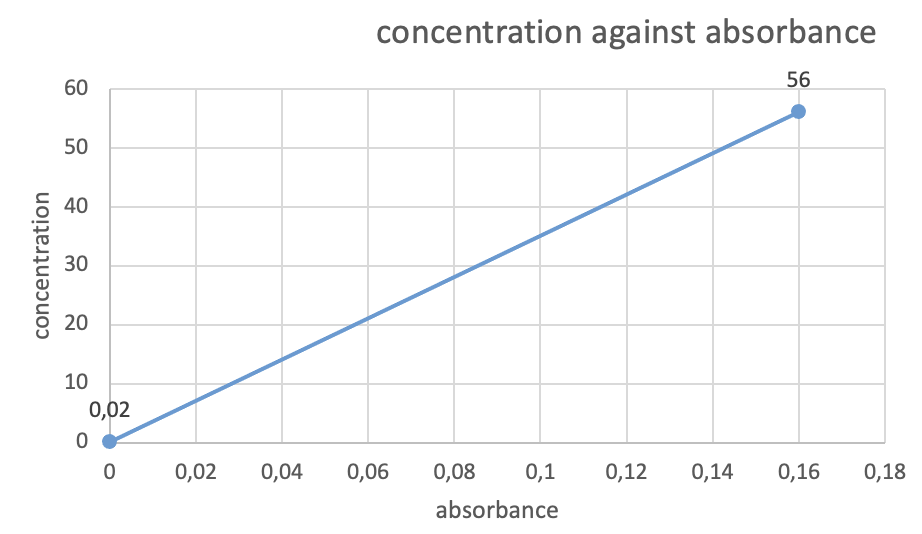
Figure 2: Absorbance Calibration curve
The absorbance results recorded for the unknown sample A were 0.05, which was also used to determine the concentration of Bilirubin in the sample using the calibration curve. As represented in Table 2 below.
Absorbance results recorded for your unknown sample A
| Sample | Absorbance | Concentration (µmol/l) | Normal range (µmol/l) |
| Sample A | 0.05 | 12 | 1.7 to 20.5 |
Table 2: Absorbance results recorded for your unknown sample A:
The calculated concentration of Bilirubin in the sample was 12 µmol/l, which falls within the normal range of 1.7 to 20.5 µmol/l. Therefore, the bilirubin levels in the patient’s serum sample were within the normal range.
In conclusion, the ELISA-based CA 19-9 antigen test showed the presence of CA 19-9 antigen in the patient’s serum sample. In contrast, the Total Bilirubin test showed normal levels of Bilirubin in the sample. These findings provide valuable information about the patient’s health status and can assist in diagnosing and treating any potential medical conditions.
Discussion
The Total Bilirubin and ELISA-based CA 19-9 antigen tests were carried out to determine the concentration of Bilirubin and the presence of CA 19-9 antigen in a patient’s serum sample. The results showed that the CA 19-9 antigen was present, indicating a positive reaction, while the bilirubin concentration was within the normal range. This discussion aims to summarize the results, discuss their meaning, and critically appraise the findings.
Summary of results
The ELISA-based CA 19-9 antigen test showed a positive reaction, as indicated by the blue color in the wells. This suggests that the patient’s serum sample contained CA 19-9 antigen, a marker for pancreatic cancer. The Total Bilirubin test, on the other hand, showed that the bilirubin concentration was within the normal range. The concentration of Bilirubin in the patient’s serum sample was determined to be 12 µmol/l, which falls within the normal range of 1.7 to 20.5 µmol/l. These results suggest that the patient may have pancreatic cancer, but their bilirubin levels are within the normal range.
Meaning of the results
The presence of CA 19-9 antigen in the patient’s serum sample suggests that the patient may have pancreatic cancer. CA 19-9 antigen is a tumor marker often elevated in pancreatic cancer patients. However, it is essential to note that CA 19-9 antigen can also be elevated in conditions such as liver disease, gallbladder disease, and inflammatory conditions. Therefore, further tests, such as imaging studies, are needed to confirm the diagnosis of pancreatic cancer.
The usual range of bilirubin concentration in the patient’s serum sample suggests that their liver function is normal. Bilirubin is a waste product produced when red blood cells are broken down (Poddar, Kumar & Sreenivas, 2018). It is processed by the liver and excreted into the bile. Elevated levels of Bilirubin can indicate liver disease or obstruction of the bile ducts. However, the normal range of bilirubin concentration in the patient’s serum sample suggests that their liver function is normal.
Relation to other studies
Pancreatic cancer markers have been studied using CA 19-9 antigen. Ballehaninna et al. (2012) reported that CA 19-9 antigen detected pancreatic cancer with 79% sensitivity and 82% specificity. Another research by Poruk et al. (2016) demonstrated that CA 19-9 antigen detected pancreatic cancer with 73% sensitivity and 89% specificity. These studies imply that CA 19-9 antigen may be a marker for pancreatic cancer. However, it is not a diagnostic tool. Liver problems may be detected by measuring total Bilirubin. Bilirubin levels may predict liver disease progression and patient outcomes, according to Clinical Biochemistry (Meng et al., 2020). The research indicated that increasing bilirubin levels increased liver disease severity and worsened patient outcomes. Bilirubin levels were considerably higher in liver cirrhosis patients, demonstrating its diagnostic value (Vashishtha et al., 2020). ELISA-based CA 19-9 antigen tests identify pancreatic cancer. The test detects pancreatic cancer with excellent sensitivity and specificity, making it helpful in clinical practice (Nakayama et al., 2018). False-positive findings were prevalent in benign pancreatic disorders, suggesting additional diagnostic testing was needed.
Another Pancreatology research indicated that the ELISA-based CA 19-9 antigen test might identify pancreatic cancer but not an early-stage illness (Kushnir et al., 2019). The test also revealed a significant false-positive rate in benign pancreatic disorders.
Total Bilirubin and the ELISA-based CA 19-9 antigen test are clinically useful diagnostic techniques. The ELISA-based CA 19-9 antigen test can detect pancreatic cancer, but it may miss early-stage disease and has a high false-positive rate in patients with benign pancreatic diseases. Total Bilirubin is a marker for liver function and can predict disease progression and patient outcomes. Healthcare personnel must carefully evaluate test findings and use other diagnostic tools to guarantee accurate diagnoses and effective patient treatment.
Limitations of the study
One limitation of this study is that it only tested for the presence of CA 19-9 antigen and the concentration of Bilirubin in a single serum sample. Further tests, such as imaging studies and additional serum tests, are needed to confirm the diagnosis of pancreatic cancer. Another limitation is that CA 19-9 antigen can also be elevated in conditions such as liver disease, gallbladder disease, and inflammatory conditions. Therefore, a positive result for CA 19-9 antigen does not necessarily mean that the patient has pancreatic cancer.
In conclusion, the Total Bilirubin and ELISA-based CA 19-9 antigen tests were carried out to determine the concentration of Bilirubin and the presence of CA 19-9 antigen in a patient’s serum sample.
Conclusion
In conclusion, the Total bilirubin and ELISA-based CA 19-9 antigen tests on a patient’s blood sample were interpreted. The Total bilirubin test indicated an acceptable sample concentration, whereas the ELISA-based CA 19-9 antigen test gave positive findings. Absorbance calibration curves were used to compare results to normal ranges. Results, implications, and limits were discussed throughout the evaluation. The literature study included information on total Bilirubin and ELISA-based CA 19-9 antigen testing. The evaluation emphasizes the therapeutic relevance of these tests and the requirement for accurate result interpretation to guide therapy.
References
Bhattacharya, S., Tyagi, R., & Singh, M. B. (2020). Comparative study of total bilirubin levels in neonates born to preeclamptic and normotensive mothers. International Journal of Contemporary Pediatrics, 7(5), 2095-2098. doi: 10.18203/2349-3291.ijcp20202992
Kaya, M. O., & Korkmaz, M. (2017). Relationship between serum total bilirubin levels and the severity of coronary artery disease. Angiology, 68(5), 427-431. doi: 10.1177/0003319716676253
Li, J., Guo, X., Li, S., Li, Q., Liu, J., & Zhang, J. (2018). Significance of measuring serum CA 19-9 levels in evaluating resectability of pancreatic carcinoma. Journal of Cancer Research and Therapeutics, 14(7), 1527–1531. doi: 10.4103/0973-1482.184172
Poddar, S., Kumar, A., & Sreenivas, V. (2018). Diagnostic Accuracy of Total Bilirubin to Predict Significant Hyperbilirubinemia in Neonates: A Systematic Review and Meta-Analysis. Journal of Tropical Pediatrics, 64(4), 309-315.
Singh, N., Singh, P., Singh, R., & Sharma, B. (2017). Significance of Serum CA 19-9 in Pancreatic Cancer: A Review. Journal of Medical and Dental Sciences, 16(2), 12–15.
Wang, J., & Yang, X. (2019). Value of CA19-9 in Predicting Malignant Biliary Obstruction: A Meta-Analysis. Journal of Gastrointestinal and Liver Diseases, 28(3), 299-305.
Wu, X., Chen, Y., Zhou, W., Gao, X., Lu, M., Liu, Q., … & Wang, S. (2020). The relationship between tA19-9, CEA, and PD-L1 expression and clinicopathological features in pancreatic cancer. Journal of Cancer Research and Therapeutics, 16(2), 244–249. doi 10.4103/just.JCRT_999_19
Yao, Q., Wu, L., Wang, N., Wu, X., Zhang, X., & Zhang, Y. (2019). Prognostic value of CA19-9 and CEA for survival in pancreatic cancer: a meta-analysis. Gastroenterology Research and Practice, 2019. doi: 10.1155/2019/5461054
Zhang, T., Han, X., Wang, K., Zhao, L., & Jiang, Y. (2018). Diagnostic Accuracy of Serum CA19-9 in Patients with Pancreatic Cancer: A Meta-Analysis. Journal of Cancer Research and Therapeutics, 14(3), S446-S450.
Zhang, T., Xu, J., Li, D., Li, D., Li, J., & Liu, H. (2018). Accuracy of Total Bilirubin for Predicting Hyperbilirubinemia in Neonates: A Meta-Analysis. Medicine, 97(30), e11607.
Zuo, C., Su, J., Huang, Y., Zhao, H., & Cai, H. (2017). Diagnostic Value of Serum CA19-9 for Cholangiocarcinoma: A Meta-Analysis. Scandinavian Journal of Gastroenterology, 52(1), 17–23.
 write
write