Abstract
2-propenamide, often known as acrylamide (AA), is an industrial compound produced in several foods, especially carbohydrate-rich foods, and asparagine processed at high temperatures. AA is a potentially carcinogenic substance that poses significant health risks for humans, especially infants, due to their unique physiological traits. Moreover, infants have higher resting metabolic rates, resulting in increased sensitivity to potentially hazardous substances. The history of acrylamide in food can be traced back to 2002 when Swedish scientists discovered that high chemical levels were present in certain foods. In-depth investigations of the existence, chemistry, food sources, and toxicology of acrylamide were launched in response to the April 2002 report of the Swedish National Food Administration announced that it was primarily present in wild foods high in carbohydrates. These studies were undertaken to determine whether the existence of this pollutant in human food posed any potential health risks. Over the past years, there has been a notable increase in the number of review articles detailing the Possibilities of acrylamide in food causing health complications. Additionally, the investigation of AA in various foods has been increasing exponentially. However, the detailed review of acrylamide in food and baby food has only been discussed on a limited basis. This review, therefore, explores the history, chemical structure, synthesis, properties, toxicity, potential health risk in the human diet and mitigations, and regulations surrounding acrylamide in food and baby food.
Keywords: Acrylamide, toxicity, asparagine, food, health risk
Introduction
Acrylamide, also known as 2-propenamide, is an industrial chemical that develops during the heating process of starchy foods [1]. It is a monomer, and a polymer manufactured commercially by hydrolyzing acrylonitrile with nitrile hydrase. A mechanism known as the Maillard reaction causes acrylamide to naturally occur when some foods, especially starchy foods like potatoes, crisps, coffee, and cereals, are cooked at high temperatures (over 120°C or 248°F) [2]. It is used to make polyacrylamide polymer, which has a wide range of applications, including electrophoresis gels, grouting agents for tunnel and dam construction, coagulants for wastewater treatment, and clarifiers for drinking water [3]. Based on its ability to cause animal cancer, acrylamide is listed by IARC as a potential human carcinogen (Group 2A). The WHO Consultation in 2002 approved this classification. SNFA first noted the presence of acrylamide in foods in 2002 [4]. Following these discussions, WHO and FAO, as well as JIFSAN/NCFST workshop, reviewed all international acrylamide information and listed several potential risks. These concerns can be overcome to better assess the health risks of this unexpected result [5]. Acrylamide, a neurotoxic monomer, has been identified in food. The original data on acrylamide levels showed no clear pattern, except that carbohydrate-rich diets produced more [2]. Food processing involves sophisticated thermal procedures. It is regarded as one of the significant heat-induced process pollutants primarily generated in potato, cereal, and pastry products. A large part of how people are exposed to acrylamide is their diet. Since recognizing high levels of acrylamide in high-temperature-cooked food, acrylamide has been thoroughly investigated [4]. The presence of acrylamide in food has caused worry even though it is widely utilized in various industries due to its possible health hazards for specific cancers, such as breast, ovarian, and endocrine-related cancers. It has been discovered that various infant foods, including cereals and crackers, have significant levels of acrylamide [6]. Baby food is not immune to the creation of acrylamide [7]. Babies are more likely than adults to acquire serious illnesses due to AA because of their various physiological characteristics, such as improved ventilation, body-surface area, and food consumption per kilogram.
Additionally, newborns are more sensitive to potentially hazardous substances because they have higher resting metabolic rates than adults, necessitating urgent removal [7], [8]. Baby food formulation, processing, and storage are crucial for the foods’ physicochemical makeup and nutritional value. According to the American Academy of Pediatrics’ recommendations to the Food and Drug Administration, infant formula must contain several nutrients. It is also required to define upper limits for each nutrient in the formula [9]. On the other hand, baby foods or infant formulae may contain a number of pollutants that pose a risk to a child’s health throughout the first few years of life [7]. This review thus focuses on acrylamide in foods and baby foods.
The history of acrylamide
Acrylamide’s presence in food dates back to 1997 when cows in southern Sweden’s rural Bjare peninsula began exhibiting frightening symptoms such as staggering, falling, and dying. The investigation results showed that the cattle had been consuming water laced with AA, which had been leaking from drilling operations on a nearby mountain tunnel. (http://www.newscientist.com/article/mg19025483.600-acrylamide-the-food-scare-the-world-forgot.html?full=true). SNFA and academics from Stockholm University published their results that AA is produced by a variety of foods that are heated during preparation or cooking, a hazardous and possibly cancer-causing chemical, in April 2002[1], [2], [6]. Since then, several studies and investigations have explored its existence in some foods. The recognition of acrylamide in food raised concerns about its potential health effects and contributed to the development of new for detecting and measuring its presence in food products [10].
Chemical Structure of acrylamide
A polyacrylamide, acrylamide (C3H5NO), is an organic compound with a linear chain of carbon and nitrogen atoms as its chemical makeup. The amide group and a conjugated vinyl group, CH2=CH—C—NH2, make up the two main functional groups of the acrylamide molecule [11].
Physical and Chemical properties of acrylamide
Acrylamide is a white, crystalline solid with a molecular weight of 71.08, a melting point of 84.5 °C, and a tendency to sublime even at ambient temperature. It is highly soluble in water, polar solvents, and organic solvents and reacts with compounds that contain hydroxyl, amino, and sulfhydryl groups (acetone, ethanol, and methanol) [1], [8], [11]. Acrylamide is stable in solution and does not polymerize on its own. An amide and vinyl group are both present in the molecule of acrylamide (CH2=CH—C—NH2) [4]. At the 3 -position, it is easy to carry out addition reactions between acrylamide and molecules containing active hydrogen atoms, such as thiols, hydroxy, and amino groups. This is explained by the amide group’s capacity to draw electrons. A basic catalyst is usually needed for the carboxymethylation reaction type. Thiols, and particularly amino groups, become much more reactive in the absence of a catalyst. To make polyacrylamide, the double bond can be polymerized via a vinyl-like method (C3H5NO) n. [12]
Uses of Acrylamide
One of the main applications for acrylamides is in producing paper, contact lenses, some dyes, chemicals, and fabrics, as well as in mining and mineral processing processes. It is used to make polyacrylamide polymer, which has a wide range of applications, including electrophoresis gels, grouting agents for tunnel and dam construction, coagulants for wastewater treatment, and clarifiers for drinking water [13].
Mechanisms of Formation of acrylamide
When certain foods, especially starchy ones, are cooked at high temperatures (over 120°C or 248°F), a process known as the Maillard reaction occurs that results in the chemical acrylamide being produced [14]. When heat is applied, a complicated chemical reaction involving reducing sugars and amino acids occurs, which is also the cause of the browning and flavor development in many cooked meals [15]. The amino acid asparagine is thought to combine with reducing carbohydrates like fructose or glucose to generate acrylamide when exposed to heat. The kind of food being cooked, the temperature and length of cooking, and the presence of other food ingredients like salt and baking powder all impact the development of acrylamide [3]. Figure 1 illustrates the primary pathways via which acrylamide forms in foods.
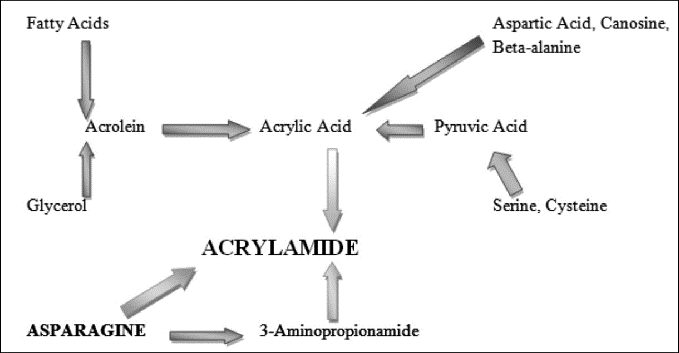
Figure 1: Primary pathway for the formation of acrylamide [16]
Formation via Asparagine Route
One of the most well-known mechanisms is asparagine-mediated acrylamide production [14]. This mechanism is based on the interaction of the amino acid asparagine with high-temperature lowering carbohydrates like glucose and fructose. This process states that heat induces an asparagine rearrangement reaction that forms an acrylamide precursor molecule, which then goes through different reactions to become acrylamide [6]. The Maillard reaction, which also causes browning and taste development in many cooked foods, is thought to be the main mechanism of this process [17]. The synthesis of AA via the asparagine route is shown in Figure 2.
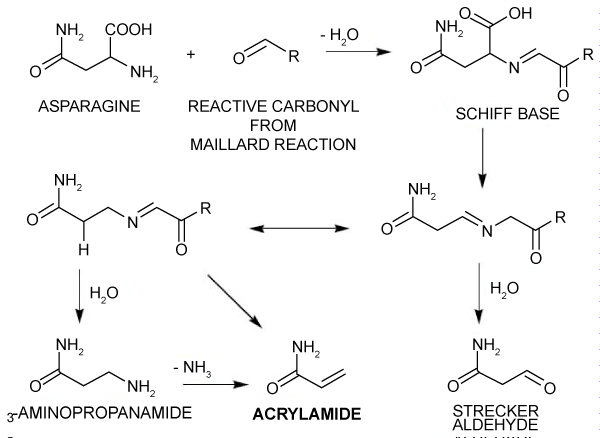
Figure 2: Proposed process for acrylamide production in heat-treated foods [6]
Biotransformation
Absorption
Animal studies have shown that acrylamide is rapidly absorbed by the dermis and mucosal tissues when breathed. Because of its water solubility can diffuse uniformly throughout the body if given orally [6].
Distribution
The dose or administration route has no significant influence on tissue distribution. Erythrocytes have the largest concentrations. Despite the prevalence of neurological consequences, acrylamide does not accumulate in the tissues of the nervous system. Acrylamide easily penetrates the placenta [6].
Metabolism
Following ingestion, AA is quickly and fully incorporated by the gastrointestinal tract and circulated to the peripheral organs in rats [13]. AA affects humans and rodents similarly [18]. An exploratory study in healthy volunteers found that blood-placenta and blood-breast milk barriers can both be penetrated by AA in vitro and in vivo in nursing women [13]. These studies suggest AA can permeate any human tissue. At least two pathways metabolize AA after consumption. CYP450 can convert it to glycinamide, which is more reactive than the parent AA molecule toward proteins and DNA, or glutathione-S-transferase can conjugate it to N-acetyl-S-(3-amino-3-isopropyl) cysteine (GST). Glutathione (GSH) conjugation by GST detoxifies AA and glycinamide. Mercapturic acids excrete GSH adducts in urine [6], [7], [13], [18]. The primary metabolites of AA are mercapturic acids and glycinamide. Their urine excretion levels may indicate AA exposure. AA and glycinamide form adducts with hemoglobin’s amino acids and DNA, indicating AA exposure [13]. AA is a dietary contaminant since feeding rats fried meals increased hemoglobin adduct levels in 2002. AA can also breach the placental barrier, despite infant blood AA-hemoglobin adducts [15].
Excretion
Acrylamide is metabolized mostly through glutathione S-transferase conjugation with decreased GSH [19]. The amount of GSH in the liver can be reduced by conditions including undernutrition, oxidative stress, and liver disease (including cirrhosis, alcoholic hepatitis, and other malignant liver illnesses). Higher acrylamide toxicity in children may be expected, especially under these circumstances, as a child’s liver cannot handle as much stress as an adult’s. Mercapturic acid conjugates are primarily excreted in the urine during elimination [20]. More than 90% of the ingested acrylamide is eliminated as metabolites in the urine. Only 2% of acrylamide is excreted unaltered. The bile and feces expel fewer amounts. Within 24 hours, 60% of a dose administered manifests in the urine [19].
Toxicity of acrylamide
When acrylamide is ingested through various channels, there are numerous health hazards. The mother’s smoking is one of the main ways the newborn is exposed to acrylamide [13]. Water contamination and contaminated food are two other common ways to expose an infant or child to acrylamide [2]. Acrylamide intake levels must be closely managed, especially in infant formulae and baby foods, as children, particularly those under the age of two, are the most susceptible group. On the other hand, mothers must be careful when selecting the water they use to cook food for their children. Acrylamide health hazards can be divided into the following categories [13].
Neurotoxicity
One of the main effects of AA intake is neurotoxicity, and studies in this field are popular [6]. In rats and humans, this chemical is neurotoxic. In investigations on rodent toxicity, single doses of 100–200 mg/kg killed most of the animals, whereas repeated doses of 10–50 mg/kg bw/d AA rendered the majority of lab mice neuropathic [2]. In vitro, AA accelerates mitochondrial malfunction and mortality in rat primary astrocytes and BV-2 microglial cells. [14] AA prevented human neuroblastoma and glioblastoma cell formation, which impacted the neurological system. Numerous epidemiological studies have shown that workers exposed to acrylamide are neurotoxic. Human neurotoxicity symptoms often include ataxia, skeletal muscle weakness, weight loss, distal edema, and axon degeneration in the central and peripheral nervous systems. Peripheral neuropathy was detected in Swedish tunnel workers exposed to quick, high-intensity doses of a grouting chemical containing AA and N-methyl acrylamide [13]. Biomarkers such as peripheral nervous system symptoms and hemoglobin adducts demonstrated a strong dose-response relationship [21].
Genotoxicity and Carcinogenicity
Many studies examined AA and its major metabolite, glycinamide, for genotoxicity [21]. In his investigation on mice, Alzahrani found that single doses of AA at 10, 20, and 30 mg/kg and repeated doses of 10 mg/kg for 1 and 2 weeks significantly damaged DNA as seen by an increase in micronuclei and chromosome abnormalities in mouse bone marrow cells [22]. Animals, both male, and female, that have been exposed for a long time to a lot of AA in their drinking water develop a lot of tumors. Animal studies have led the EFSA to conclude that AA in food may raise cancer risk in all consumers, particularly in children who are most exposed [23]. Even though AA is carcinogenic to experimental animals, few epidemiologic investigations on workplace and dietary exposure to AA have discovered evidence of its impact on human cancer. IARC and US NTP classifications of AA in the most recent study include “probable human carcinogen” and “reasonably projected to be a human carcinogen.” [7]
Reproductive Toxicity
The No-observed negative impact limit for rats was established to be 2 to 5 mg/kg/d, even though the toxicity of AA on human reproduction has not been established [2]. After ingesting AA dosages of 0.5 to 10 mg/kg, rats’ development slowed, and their epididymal sperm reserves were lower than in the control group [6]. Additionally, following 20 days of daily injections of AA (20 mg/kg) into male rats, testosterone and prolactin levels dropped in a dose-dependent way. According to a different experiment, female mice treated with AA were also found to have reproductive toxicity. The amount of corpora lutea, progesterone production, and mouse granulosa cell viability were all observed to be declining [22].
Hepatotoxicity
Although AA is processed in the liver, only a small percentage of users have liver damage. But numerous animal studies have demonstrated how dietary AA damages the liver due to oxidative stress [23]. The liver GSH levels and overall antioxidant status of experimental adult rats were significantly reduced after receiving a high dose of 25 mg/kg AA for 21 days [22]. The administration of AA also increased the blood levels of the liver enzymes AST, ALT, and ALK, a decrease in catalase and superoxide dismutase activity, and an increase in malondialdehyde and total oxidant status [13].
Immunotoxicity
Research on the harmful effects of AA on the immune system is a little extensive [24]. However, AA immunotoxicity was discovered in female BALB/c mice. It led to pathological alterations in the spleen, thymus, and lymph glands and decreased their final weights and lymphocyte counts [22]. No links between prenatal dietary exposure to AA and the investigated immune-related health outcomes or blood indicators at any age have been discovered, despite the fact that it has been demonstrated that acrylamide can cross the placenta and enter the fetus [13].
Mechanism of AA Detoxification
If AA undergoes CYP450 processing and transforms into glycinamide or bonded to the antioxidant glutathione, it can be detoxified in the body after consumption [6]. It is nonetheless possible to expose oneself to excessive amounts of AA and surpass these pathways’ capacity for AA detoxification despite the metabolic pathways’ assistance for this process. Consuming foods high in cysteine, an important substrate for the synthesis of glutathione, such as onions, garlic, cruciferous vegetables, and red peppers as well as foods high in sulfur, such as broccoli, Brussels sprouts, and onions, can help increase glutathione levels, which may reduce the risk of toxicities from AA [1]. The amino acid cysteine is significant in foods like poultry, yogurt, and eggs [6].
Acrylamide in food
Raw food products do not naturally contain harmful substances; instead, these substances are created during the high-temperature heat processing of foods high in carbohydrates [4]. Because of the availability of natural reactants, including glucose, fructose, and asparagine, foods made from plants are a major source of acrylamide [2]. A thorough investigation of acrylamide exposure evaluation in food led to determining the level of acrylamide in foods. Table 1 lists the acrylamide ranges for various dietary categories.
| Product/Product group | AA range |
| Bakery products and biscuits | 18-3324 |
| Bread | <10-3200 |
| Cereals | <10-1649 |
| Chocolate products | <2-826 |
| Coffee substitute | 80-5399 |
| Dairy products | <10-130 |
| French fries/chips | 59-5200 |
| Meats | <10-116 |
| Potatoes (raw) | < 10-<50 |
| Potato chips/ crips | 117- 4215 |
Table 1: Quantity of acrylamide in various foods and food product groups [6]
Estimation techniques for acrylamide in food
Chromatographic procedures provide modern analytical chemistry and fast, precise, and accurate results. Researchers from different nations were alerted to the acrylamide concerns made in 2002 in order to confirm its quantity and presence in food [25]. Techniques frequently used include LC-MS and GC-MS using isotope-labeled internal standards. GE-MS, LC-MS, HPLC-MS, GC-MS, and electrophoresis using non-aqueous capillaries are a few of the analytical techniques used to identify acrylamide [6]. Using solid-phase extraction to eliminate interference-causing chemicals, LC-MS can detect acrylamide. Using LC-MS prevents derivatization and provides uniform matrix and ion suppression. UPLC-MS/MS with TOF determined acrylamide, its intermediates, and its kinetic properties. LC-MS analysis found 8.974 and 0.151 gkg1 of acrylamide in cookies and mashed potatoes, respectively. Potato chips and French fries had 0.50–9.250 and 0.02 g/kg acrylamide, respectively. The other method for acrylamide detection is UPLC-electrospray-MS/MS, which takes 3 min and has good repeatability [26]. GC-MS and this technique have become increasingly popular due to the low molality and polarity induced by adding bromine to the olefin molecule [25]. For analytical selectivity, GC-MS analysis uses FID or ECD and MS. With a LOD of 0.01 gkg1, GC and ECD can quickly identify acrylamide in potato chips and fries. HPLC-MS is another sensitive method for food acrylamide analysis. HPLC-MS/MS found LOD to be 0.038 mg kg1 and LQ to be 0.004 mg kg1 for food and drinks. Solid meal acrylamide LOD ranged from 1.480 mg kg to 1. [27] CE, and mass detection can identify organic food contaminants. CZE and FASI showed linear calibration curves with 0.003 mg kg1 LOD. FASI and CE/MS detected acrylamide at 0.008 mg kg1. FASI-CZE was exclusively used to assess acrylamide in biscuits, cereals, crispbread, and coffee due to its low LOD. Fluorescent sensing can detect acrylamide using quantum dots. Linear range and LOD were 35-350 000 and 35 gkg1, respectively [28]. The electrochemical approach had higher sensitivity limitations than the fluorescence method. AA determination takes time and money. Therefore, researchers today seek fast and economical AA determination solutions. Bakery products’ AA content can be estimated by correlating color values like browning and HMF content with the AA value. Only heat-sensitive items, such those used in bakeries, can be employed with this predictive technology [3].
Mitigation Strategies for Acrylamide
To limit the amount of acrylamide in foods, significant attempts have been made to develop suitable solution. The public can be protected from food dangers and can have their perceptions of food safety improved by reducing the concentration of acrylamide in foods at the household and industrial levels [29]. The below mentioned are a number of techniques to mitigate acrylamide that have been investigated so far in relation to acrylamide generation at various points in the food production process.
Effect of Raw Materials
Acrylamide formation in potato and cereal products depends on variety, harvest year, fertilization, and storage conditions [5]. The high reducing sugar content of potatoes offsets the beneficial effect of cereal variety on precursor and acrylamide concentrations by limiting acrylamide production in potato products. Thus, limiting reducing sugars and asparagine may minimize acrylamide in potato and cereal goods, respectively. Asparagine and reducing sugars in potatoes are affected by climate. Crop production depends on fertilization. A decrease in nitrogen fertilization increased reducing sugar concentrations, which increased acrylamide production in potato goods but not in bakery items. Wheat’s reducing sugars were unaffected by fertilization. Potato tubers are preserved for months to provide a year-round supply [6]. Cold temperatures and senescent sweetening cause potatoes to accumulate sugar during storage. Potato sprouting is linked to senescent sweetening caused by storage above 8°C. Low temperatures (below 8°C) inhibit sprouting and reduce sugar accumulation. Potatoes stored at 8°C have no significant changes in reducing sugars or asparagine content. Potatoes should be stored at 8°C to prevent acrylamide formation [1].
Effect of Additives
Dietary acrylamide synthesis is decreased by asparaginase, an enzyme that converts the precursor asparagine into ammonia and aspartic acid. It is mostly utilized in potato and grain products and is commercially produced from Aspergillus niger (DSM’s Preventase) or Aspergillus oryzae (Novozyme’s Acrylaway) [2]. It’s expensive but promising for acrylamide reduction. Amino acids or protein-rich meals lower acrylamide. Glycine, cysteine, methionine, glutathione, and lysine were studied for their effects on acrylamide production and removal. Cysteine and methionine in cracker and potato dough reduced acrylamide formation by 50%. Flückiger and Salih found no influence of cysteine on crisp bread acrylamide production [4]. Antioxidants affect the Maillard reaction, which produces acrylamide. Rosemary, bamboo, and green tea antioxidants can lower acrylamide in cooked foods. Acrylamide may interact with active aldehydes and prevent acrolein oxidation, but the specific process is unknown. Most of these studies are small-scale or in vitro, therefore they might not be applicable in a commercial or industrial settings. In dough, mono- and divalent cations (Na+ and Ca2+ or Mg2+) dramatically reduced acrylamide. Polyvalent cations inhibit acrylamide production during heating [6]. These ions may inhibit asparagine from forming the Schiff base intermediate and acrylamide. Several investigations found that NaCl polymerization reduced acrylamide. Higher NaCl levels increased acrylamide due to yeast growth inhibition. Alginic acid and pectin reduced acrylamide synthesis in potato strips, but carob gum, carrageenan, hydroxypropyl distarch phosphate, and xanthan gum increased it [4].
Effect of the Processing Conditions
Most acrylamide-reduction strategies target processing. Blanching, frying, and heating duration affect acrylamide production [1]. Several studies indicated that baking temperature and time strongly correlated with acrylamide production. However, prolonged baking (260°C, 20 min) reduced food acrylamide. Acrylamide was also produced below 100°C by Biedermann and Grob. Optimized baking conditions reduced acrylamide production by 50%. Convection baking ovens reduce acrylamide less than conduction and radiation. Conventional and dielectric (microwave) heating reduced acrylamide in baked products [1], [4], [6]. High humidity baking reduced acrylamide in baked items. Reduce the temperature and use steam to finish baking [164]. 200°C for 20 min was the baking reference. Acrylamide production is largely in the outside crust of bread and barely in the crumb. Predicting French fry creation requires blanching. Before frying, leaching the precursors (reducing sugars) lowers acrylamide production. Blanching temperature and time can be adjusted to maintain product standards [30]. French fries and potato chips had 65% and 96% less acrylamide after blanching at 70°C for 10–15 min. Industrial and domestic food processors fry. Acrylamide production is linked to color development in Maillard reaction, particularly at the end of frying. Intensive frying produces darker fries and more acrylamide. Thus, time and frying temperature (should not exceed 170-175oC) reduce acrylamide. Vacuum-frying reduces acrylamide [6].
PH, Water Activity, and Fermentation Effects
Maillard process strongly affects pH. High pH affects dietary nutrition. Researchers found that lowering pH during frying and baking significantly lowers acrylamide. Acids lower food pH and generate Maillard compounds. Acids reduced acrylamide in corn chips, semi-finished biscuits, and cracker models [6]. Maillard processes diminish acrylamide production and pH. Food moisture reduces acrylamide production [1]. Acrylamide formed in food only at water activity below 0.8, however it is high at 0.4 and below. Water movement improves acrylamide elimination from hot foods like biscuits and potato chips [7]. Precursor composition and pH affect food acrylamide production during fermentation. Bread and fried potato products reduced acrylamide with at least an hour of fermentation [10]. Potato products reduced acrylamide by lactic acid fermentation and blanching [13].
Legislative Requirements Regarding Acrylamide Levels in Food
Given that AA is a pollutant and a chemical risk in the food industry as a compound harmful to health [23], the European Union (EU) Commission came to the conclusion that it was necessary to monitor its presence in food and foodstuffs in all EU countries (Article 2 Regulation No 178/ 2002) [13]. This monitoring was focused on the foods that either contained a large amount of AA or had high AA concentrations. The Commission recommended collecting reliable data on AA levels in food over at least three years across the Community to understand the levels of AA in foodstuffs known to contain high AA levels and/or contribute significantly to the dietary intake of the general population and vulnerable groups like infants and young children [31]. Thus, Member States should monitor AA levels in foods included in Annex 1 of Recommendation [30]. To compile a single database, Member States should send the EFSA monitoring data from the previous year by June 1 in the format and details described in Annex II. www.fsai.ie/legislation/food/contamination/acrylamide.html. International attempts to create strategies for AA reduction are progressively becoming successful. Furthermore, FDA is aware that several US firms are looking at approaches to lower the amount of AA in their products.
Conclusion
The SNFA’s April 2002 research on elevated AA levels in carbohydrate-rich foods processed at >120C raised global health concerns. This declaration prompted extensive research into AA, its food presence, and its health risks. The FDA and WHO consider AA a food-borne toxin. Many research have described the health consequences of AA, but few have discussed ways to minimize AA in processed food. This review analyzed the history, chemical structure, synthesis, properties, toxicity, potential health risk in human diet and mitigations as well as regulations surrounding acrylamide in food and baby food.
Abbreviations
AA: Acrylamide
ALK: Anaplastic lymphoma kinase
ALT: Alanine transaminase
AST: Aspartate aminotransferase
CE: Capillary Electrophoresis
CZE: Capillary Zone Electrophoresis
DNA: Deoxyribonucleic Acid
ECD: Electron Capture Detection
EFSA: European Food Safety Authority
FAO: Food and Agriculture Organization
FASI: Field Sample Injection
FID: Flame Ionization Detection
GC-MS: Gas Chromatography-Mass Spectrometry
GE-MS: Capillary Electrophoresis Mass Spectrometry
HPLC-MS: High-Performance Liquid Chromatography-Mass Spectrometry
IARC: International Agency for Research on Cancer
JIFSAN/NCFST: Joint Institute for Food Safety and Applied Nutrition/National Center for Food Safety and Technology
LC-MS: Liquid Chromatography-Mass Spectrometry
LOD: Limit Of Detection
LOQ: Limit Of Quantitation
SNFA: Swedish National Food Administration
TOF: Time Of Flight
UPLC-MS/MS: Ultra-Performance Liquid Chromatography-tandem Mass Spectrometry
US NTP: US National Toxicology Program
WHO: World Health Organization
References
[1] L. Rifai and F. A. Saleh, “A Review on Acrylamide in Food: Occurrence, Toxicity, and Mitigation Strategies,” Int. J. Toxicol., vol. 39, no. 2, pp. 93–102, 2020, doi: 10.1177/1091581820902405.
[2] L. Rifai and F. A. Saleh, “A review on acrylamide in food: occurrence, toxicity, and mitigation strategies,” Int. J. Toxicol., vol. 39, no. 2, pp. 93–102, 2020.
[3] Y. Tepe and A. Çebi, “Acrylamide in environmental water: a review on sources, exposure, and public health risks,” Expo. Heal., vol. 11, pp. 3–12, 2019.
[4] N. Khorshidian, M. Yousefi, M. Shadnoush, S. D. Siadat, M. Mohammadi, and A. M. Mortazavian, “Using probiotics for mitigation of acrylamide in food products: a mini review,” Curr. Opin. Food Sci., vol. 32, pp. 67–75, 2020.
[5] M. Lambert, C. Inthavong, F. Hommet, J.-C. Leblanc, M. Hulin, and T. Guérin, “Levels of acrylamide in foods included in ‘the first French total diet study on infants and toddlers,’” Food Chem., vol. 240, pp. 997–1004, 2018.
[6] K. T. Visvanathan R, “Acrylamide in Food Products: A Review,” J. Food Process. Technol., vol. 05, no. 07, 2014, doi: 10.4172/2157-7110.1000344.
[7] F. Esposito et al., “Acrylamide in baby foods: a probabilistic exposure assessment,” Foods, vol. 10, no. 12, p. 2900, 2021.
[8] C. P. Boyaci-Gunduz, “Acrylamide exposure of infants and toddlers through baby foods and current progress on regulations,” Curr. Opin. Food Sci., vol. 46, p. 100849, 2022.
[9] B. Başaran and F. Aydın, “Determination of acrylamide levels in infant formulas and baby biscuits sold in Turkey,” Lett. Appl. NanoBioScience, vol. 11, pp. 3155–3165, 2022.
[10] S. Raffan and N. G. Halford, “Acrylamide in food: Progress in and prospects for genetic and agronomic solutions,” Ann. Appl. Biol., vol. 175, no. 3, pp. 259–281, 2019.
[11] G. Sennakesavan, M. Mostakhdemin, L. K. Dkhar, A. Seyfoddin, and S. J. Fatihhi, “Acrylic acid/acrylamide based hydrogels and its properties-A review,” Polym. Degrad. Stab., vol. 180, p. 109308, 2020.
[12] H. Zhang, Y. Cheng, X. Hou, B. Yang, and F. Guo, “Ionic effects on the mechanical and swelling properties of a poly (acrylic acid/acrylamide) double crosslinking hydrogel,” New J. Chem., vol. 42, no. 11, pp. 9151–9158, 2018.
[13] A. Koszucka, A. Nowak, I. Nowak, and I. Motyl, “Acrylamide in human diet, its metabolism, toxicity, inactivation and the associated European Union legal regulations in food industry,” Crit. Rev. Food Sci. Nutr., vol. 60, no. 10, pp. 1677–1692, 2020.
[14] A. A. Maan et al., “Acrylamide formation and different mitigation strategies during food processing–a review,” Food Rev. Int., vol. 38, no. 1, pp. 70–87, 2022.
[15] J. Michalak, M. Czarnowska-Kujawska, J. Klepacka, and E. Gujska, “Effect of microwave heating on the acrylamide formation in foods,” Molecules, vol. 25, no. 18, p. 4140, 2020.
[16] N. Munir et al., “L-Asparaginase potential in acrylamide mitigation from foodstuff: a mini-review,” Prog. Nutr, vol. 21, no. 3, pp. 498–506, 2019.
[17] M. Bin-Jumah, A.-F. M. Abdel-Fattah, E. M. Saied, H. R. El-Seedi, and M. M. Abdel-Daim, “Acrylamide-induced peripheral neuropathy: manifestations, mechanisms, and potential treatment modalities,” Environ. Sci. Pollut. Res., vol. 28, pp. 13031–13046, 2021.
[18] S. Zhao et al., “Comprehensive analysis of metabolic changes in rats exposed to acrylamide,” Environ. Pollut., vol. 287, p. 117591, 2021.
[19] R. Prabha and V. K. Nigam, “Biotransformation of acrylamide to acrylic acid carried through acrylamidase enzyme synthesized from whole cells of Bacillus tequilensis (BITNR004),” Biocatal. Biotransformation, vol. 38, no. 6, pp. 445–456, 2020.
[20] A. Bellicha et al., “Dietary exposure to acrylamide and breast cancer risk: results from the NutriNet-Santé cohort,” Am. J. Clin. Nutr., vol. 116, no. 4, pp. 911–919, 2022.
[21] J.-S. Park et al., “Developmental and neurotoxicity of acrylamide to zebrafish,” Int. J. Mol. Sci., vol. 22, no. 7, p. 3518, 2021.
[22] A. M. Abdel-Moneim, H. Elsawy, A. M. Alzahrani, A. Ali, and O. Mahmoud, “Silymarin ameliorates acrylamide-induced hyperlipidemic cardiomyopathy in male rats,” Biomed Res. Int., vol. 2019, pp. 1–8, 2019.
[23] M. Kopanska, R. Muchacka, J. Czech, M. Batoryna, and G. Formicki, “Acrylamide toxicity and cholinergic nervous system,” J Physiol Pharmacol, vol. 69, no. 6, pp. 847–858, 2018.
[24] E. Zamani, M. Shokrzadeh, A. Ziar, S. Abedian-Kenari, and F. Shaki, “Acrylamide attenuated immune tissues’ function via induction of apoptosis and oxidative stress: Protection by l-carnitine,” Hum. Exp. Toxicol., vol. 37, no. 8, pp. 859–869, 2018.
[25] B. Basaran and F. Aydin, “Estimating the acrylamide exposure of adult individuals from coffee: Turkey,” Food Addit. Contam. Part A, vol. 37, no. 12, pp. 2051–2060, 2020.
[26] A. Desmarchelier et al., “Towards a consensus LC-MS/MS method for the determination of acrylamide in food that prevents overestimation due to interferences,” Food Addit. Contam. Part A, vol. 39, no. 4, pp. 653–665, 2022.
[27] S. E. Kepekci Tekkeli, C. Önal, and A. Önal, “A review of current methods for the determination of acrylamide in food products,” Food Anal. Methods, vol. 5, pp. 29–39, 2012.
[28] R. Prata, M. V. Pérez, M. H. Petrarca, H. Teixeira Godoy, A. Garrido Frenich, and R. Romero-González, “Determination of acrylamide in commercial baby foods by LC-QqQ-MS/MS: a simple method for routine analyses,” Ms A Simple Method Routine Anal., 2023.
[29] N. Bachir, A. Haddarah, F. Sepulcre, and M. Pujola, “Formation, mitigation, and detection of acrylamide in foods,” Food Anal. Methods, vol. 15, no. 6, pp. 1736–1747, 2022.
[30] V. Patial, V. Kumar, R. Joshi, M. Gupta, and D. Singh, “Acrylamide mitigation in foods using recombinant L-asparaginase: An extremozyme from Himalayan Pseudomonas sp. PCH182,” Food Res. Int., vol. 162, p. 111936, 2022.
[31] M. S. Cantrell and O. M. McDougal, “Biomedical rationale for acrylamide regulation and methods of detection,” Compr. Rev. food Sci. food Saf., vol. 20, no. 2, pp. 2176–2205, 2021.
 write
write