Introduction and Rationale
The main aim of the investigation is to evaluate how different temperatures affect the rate of hydrolysis of acetylsalicylic acid in an acidic medium. Temperature is one of the key factors that alter the rate of reaction in most chemical reactions and can use to evaluate the strength of the bond present. Temperature tends to increase the kinetic energy of particles when heated, which makes the particles collide faster. The higher collision causes arise in the rate of reaction, which alters the overall yield of the reaction. On the other hand, hydrolysis can be defined as the process through which a chemical substance breaks into two molecules after the addition of water. Hence hydrolysis of acetylsalicylic acid takes place underwater and is one of the key determiners of how the drug should be stored. My interest in exploring this topic began when I discovered that temperature is another factor that affects the rate of hydrolysis of substances in an acidic medium. I have been passionate about studying the hydrolysis reactions of different substances since I joined high school, which made me develop a passion for the rate of hydrolysis. When learning about the rate of hydrolysis as a part of the Chemistry syllabus, I began thinking of how different temperatures could potentially affect the rate of hydrolysis and how this concept can be used to develop chemical substances such as medicines. Physical form and other factors, such as temperature and catalyst, affect the hydrolysis rate. Therefore, combining my knowledge of chemistry and passion for hydrolysis reactions, I decided to focus my internal assessment on the impact of different temperatures on the rate of hydrolysis of acetylsalicylic acid in an acidic medium. The outcomes of this investigation would be seductive to me since it will increase my knowledge and skills in hydrolysis reactions.
Brief theoretical background
Hydrolysis is defined as a chemical process where molecules of water are added to a substance. This addition of water molecules causes both the water and substance to split into two parts in such a way that one of the parent molecules poses a hydrogen ion. For instance, the process of hydrolysis can be experienced in the addition of sulphuric acid in the water, where bisulfate and hydronium compounds are formed. Acetylsalicylic acid is a non-steroidal inflammatory drug that is used to reduce pain. In most cases, acetylsalicylic acid is referred to as aspirin. The rate of hydrolysis is obtained by taking the inverse of the time or volume of the product formed. Time in the rate of hydrolysis is taken as the time that the acetylsalicylic acid took to dissolve completely at a given temperature. The rate of hydrolysis can be altered by factors such as temperature, concentration, and availability of catalyst. However, in this investigation, one would evaluate the rate of hydrolysis of acetylsalicylic acid under different temperatures. Acetylsalicylic acid(Asprin) is an acidic substance and, in most cases, is used as a drug to reduce pain, while water is a neutral substance used as a solvent in a chemical reaction (Pornchai et al., 2022). The hydrolysis reaction between acetylsalicylic acid and water produces 2-hydroxybenzoic acid and ethanoic acid, as shown in the following equation.
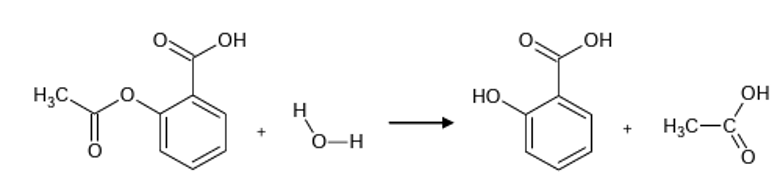
Acetylsalicylic acid(Asprin) contains both ester and carboxylic acid functional groups. It is a weak acid that is slightly soluble in water and can be prepared by reacting acetic anhydride and salicylic acid in the availability of an acid catalyst. The chemical formula for aspirin is given by the following general formula: CH3COOC6H4COOH(aq).
Evaluating the rate at which Acetlylaslicylic acid hydrolyzes has two main essential reasons. One is that aspirin hydrolyzes when administered to the body and when exposed to humid environments such as bathrooms. Hence it is vital for one to determine the appropriate temperature and rate at which aspirin hydrolyzes in order to store it in a favorable condition.
The hypothesis of the investigation
Hypothetically speaking, it is believed that an escalation in the temperature would result in an increase in the rate of hydrolysis of acetylsalicylic acid and water. It is also expected that the relationship between temperature and rate of hydrolysis of acetylsalicylic acid would be linear or directly proportional. This relationship is due to the fact that an increase in temperature causes an increment in the kinetic energy of the reacting particles(acetylsalicylic acid and hydrogen reagent), which makes them collide faster, resulting in an increase in the rate of hydrolysis (Shah et al.,2022). The control part of the experiment will have zero effect on the rate of hydrolysis since it will not be restricted with time, and some of the factors, such as mass, and concentration, will remain the same. The hypothesis would be tested by heating aspirin and water at different temperatures using an electric hotplate and later plotting the dataset obtained using excel. The constant regression approach would be used to determine the magnitude of the relationship between the temperature and rate of hydrolysis. After stating the hypothesis, it was essential to define the variables of the investigation before conducting data collection and analysis.
Variables and reason for control
The following table contains variables that were used in this investigation, the method of control, and the reason for control. The variables were essential since they enabled sufficient testing and proving of the hypothesis.
Table 1:Variables used and reason for control
| Variable Type | Control | Method | Reason for control |
| dependent variable | The temperatures for acetylsalicylic acid and water for each trial of the experiments | The temperatures were changed in the interval range of (30,40,50,60,70,80,90,100) of acetylsalicylic acid solution in the respective trials. | The variable was used to evaluate how different temperatures alter the rate of hydrolysis. |
| Independent variable | The rate of hydrolysis | The hydrolysis rate was determined by taking the inverse of the respective time obtained for the aspirin to dissolve. | This was done to evaluate how different temperatures affect the rate of hydrolysis. |
| Controlled variable | The concentration of acetylsalicylic acid (aspirin) | The concentration of acetylsalicylic acid was kept at a constant mass of 200 grams for the whole experiment. | This was done to ensure that the mass of the reactant remained the same |
| Controlled Variable | The volume of water | This was done to ensure that the volume of reactants remained the same | volume has an effect on the concentration, which increases the rate of hydrolysis |
Materials and Apparatus
The followings are the materials and apparatus that were used in this investigation :
- 250 ml of phosphoric acid
- 150ml of salicylic acid
- 250 ml of warm alcohol
- Cold water
- Warm water
- 150 mL of acetic anhydride
- 100 ml of ethanol
- Pen
- Worksheet
- Stopwatch
- Eye protection
- Beaker
- Conical flask
- Stirring rod
- Funnel
- Electric hotplate
- Erlenmeyer flask
- Crystallizing dish
- laboratory manual
Experiment setup
The following diagram illustrates the setup of the experiments that were done in order to collect the data that would be used in this investigation.

Procedure and methodology
- Assemble all the materials and apparatus in the workstation
- Read the laboratory manual
- Dry an Erlenmeyer flask first before conducting the experiments
- Pour 150ml of salicylic acid into the flask.
- Pour 250 ml of phosphoric acid together with 150 mL of acetic anhydride into the flask.
- Ensure to mix the solution and place the flask into warm water for around 10 minutes.
- Add around 30 ml of cold water to the warm solution. This addition of cold water actually destroys the excess acetic anhydride.
- Ensure to place the flask into an ice bath in order to cool the mixture and escalate crystallization.
- Use a bucker funnel to pour the mixture after finishing the crystallization process.
- Wash the crystals using ice-cold water in order to minimize the loss of the product.
- Conduct recrystallization in order to purify the product formed.
- Place the crystals in 100 ml of ethanol and Stir the mixture in order to dissolve the crystals.
- Pour 250 ml of warm alcohol into the solution formed and cover it in order to form crystals as it cools.
- Place the beaker into an ice bath once the crystals appear in order for recrystallization to occur.
- Apply suction filtration on the contents poured onto the beaker.
- Remove the excess water from the crystals formed by placing them on dry paper.
- Authentic the substance formed, whether it is acetylsalicylic acid(aspirin) through a melting point of 135°C
- Put water into an electric hot plate and add 200 grams of aspirin (acetylsalicylic acid)
- Raise the temperatures in intervals of (30°C,40°C,50°C,60°C,70°C,80°C,90°C, and 100°C) and stir the solution until the aspirin has dissolved completely
- Record the time taken for the aspirin to dissolve completely at the interval of temperatures
- Repeat the procedure for the other four trials and tabulate the temperature and time taken for aspirin to dissolve completely
- Add around four drops of phenolphthalein indicator and stir gently to mix the content. Observe the change in color
- Pour the colored solution obtained into the sink.
- Reinse all the apparatures
Safety procedure and Precaution
The concentration of 2.0 M of acetylsalicylic acid and water solution is corrosive and caustic (Trinh et al., 2022). They can cause skin burns or damage eyes if not handled with care. Ensure to put on gloves, lab coats, and goggles throughout the experiment to protect the skin and eyes from the corrosive effects of the acid and base reactants. Rinse all the apparatus and ensure to dispose of the products after the experiments have been conducted to the fullest.
Qualitative data analysis
The phenolphthalein indicator’s color changed from colorless to white due to 2-hydroxybenzoic acid and ethanoic acid forming as the end product (Mishra et al., 2022). The temperature recorded was also observed to increase due to the heat production between the two reactants. The volume of the hydrogen gas produced was also noted to increase as temperature increased.
Data collection
The following table indicates the set of data that was collected from the experiment. The main set of data that was collected was about the temperature of the solution after it was subjected to an electric hotplate. The time taken for the aspirin to dissolve was also recorded, which was fundamental in evaluating the rate of hydrolysis.
Table 2: Data collected
| Temparature of Solution(degree celcius) | Time (seconds )Trials | ||||
| 1 | 2 | 3 | 4 | 5 | |
| 30 | 42.8 | 42.5 | 42.9 | 43.7 | 43.5 |
| 40 | 40.83 | 40.6 | 40.9 | 41.6 | 40.5 |
| 50 | 38.8 | 38.6 | 38.6 | 38.9 | 39.12 |
| 60 | 36.9 | 36.7 | 37.8 | 37.63 | 36.5 |
| 70 | 32.9 | 33.5 | 32.3 | 34.9 | 34.8 |
| 80 | 30.7 | 31.8 | 30.9 | 32.7 | 30.9 |
| 90 | 28.4 | 27.7 | 28.8 | 26.7 | 28.8 |
| 100 | 24.9 | 24.6 | 24.8 | 24.9 | 24.6 |
After the data was collected, it was essential to calculate the average values of the trials. The following formulae were used to obtain the average time for the three trials in seconds:
For instance, the average time for the 30 degrees Celcius interval was determined as follows and the concept utilized to obtain the average for the other temperature ranges:
Table 3: Processed data: Average time
| The temparature of acectylsalicylic acidSolution (degree celcius) | Average time |
| 30 | 24.76 |
| 40 | 28.08 |
| 50 | 31.40 |
| 60 | 33.68 |
| 70 | 37.10 |
| 80 | 38.80 |
| 90 | 40.88 |
| 100 | 43.08 |
The rate of hydrolysis was computed by taking the inverse of the time taken for the aspirin to dissolve completely in the heated water. The Following formula was applied:
Rate of hydrolysis = 1 ÷ Time consumed for the aspirin to dissolve completely
The rate of hydrolysis at 30 degrees was calculated as follows and the concept utilized for the other temperature intervals:
Rate of hydrolysis = 1 ÷ 24,76
Rate of hydrolysis = 0.023213
Table 4: Processed data: The calculated rate of hydrolysis
| The concentration of HCL Solution (ML) | Rate of reaction |
| 30 | 0.023213 |
| 40 | 0.024458 |
| 50 | 0.025771 |
| 60 | 0.02695 |
| 70 | 0.029691 |
| 80 | 0.031847 |
| 90 | 0.035613 |
| 100 | 0.040388 |
After the rate of hydrolysis was computed and tabulated in table 2 above. The data was plotted using excel software in order to evaluate the relationship between temperature and rate of hydrolysis. The following graph was generated :
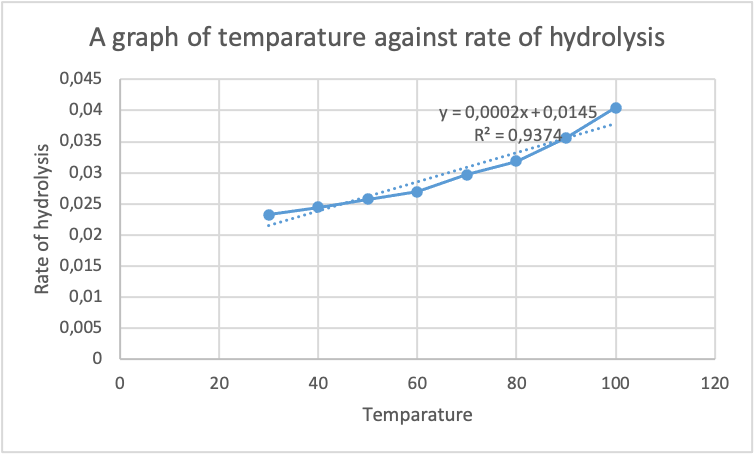
From the graph in figure 1 above, it can be evidently seen that the relationship between the temperature of the reactants of acetylsalicylic acid and water is directly proportional to the rate of hydrolysis of the products formed. An escalate in the temperature of acetylsalicylic acid results in an increment in the rate of hydrolysis of the product formed. This increase in the rate of hydrolysis is due to the fact that an increase in temperature causes an increase in the kinetic energy of the reactants. The increased kinetic energy causes the particles to move and collide faster, which results in an increased rate of hydrolysis. The regression constant obtained was 0.968, indicating a strong positive relationship between the temperature of the acetylsalicylic acid solution and the rate of hydrolysis of the product formed. This proportional relationship is due to the fact that an increase in the temperature of reactants causes an increase in the collisions of those reactants in a given period of time. Hence, the impact of temperature on the rate of hydrolysis of acetylsalicylic acid is significant.
Uncertainties
The concentration of acetylsalicylic acid was measured in two decimal places. Hence the uncertainty in the measurement was . The was measured using a graduating cylinder with an uncertainty of ± 0.05 cm³
Uncertainty of 250 cm³ of 0.1 M of acetylsalicylic acid solution
percentage uncertainty = uncertainity÷value×100
percentage uncertainty of mass used= 0.01 ÷ 200 = ± 0.005%
percentage uncertainty of volume used= 0.5 ÷ 250 × 100 = ± 0.003%
percentage uncertainty of volume used= 0.5 ÷ 250 × 100 = ± 0.003%
Therefore, the total uncertainty would be = 0.005 + 0.003 = 0.08%
The total uncertainty obtained was 0.08 % which indicates high precision during data collection. The trend line is also noted to be small with no overlapping.
Conclusion
The main aim of the investigation was to evaluate how different temperatures on the rate of hydrolysis of acetylsalicylic acid. The rate of reaction of acetylsalicylic acid and water was seen to produce 2-hydroxybenzoic acid and ethanoic acid. It was first hypothesized that an increase in the temperature of the reactant would escalate the overall rate of hydrolysis of acetylsalicylic acid. The data obtained from the experiments and graphical analysis showed that an escalation in the temperature of the reactants resulted in an increase in the rate of hydrolysis. This increase in the rate of hydrolysis was due to the fact that an increase in temperature causes an increase in the collision of the molecules within the reactants (Wilatika et al.,2022). As the molecules collide faster, they result in an increment in the kinetic energy of the reactant, which escalates the hydrolysis rate.
The high value of regression constant 0.968 obtained supported the investigation, which indicated that there is a strong relationship between the concentrations temperature and the rate of hydrolysis of acetylsalicylic acid. This relationship demonstrated that an increase in temperature causes an increase in the rate of hydrolysis. The error bars on the trend line were very small and did not overlap, suggesting a high measure of accuracy in the dataset obtained in the experimental activity. Therefore the relationship between temperature and the rate of hydrolysis in acetylsalicylic acid is significant, and thus temperature affects the rate of hydrolysis of acetylsalicylic acid.
Evaluation
The following table contains the sources of the strength of the investigation.
Table 5: Strength of the investigation
| Regression constant | The strong value of the regression constant of 0.968 was one of the strengths of the investigation, which indicated a strong correlation between the two variables. |
| The high number of trails | The high number of trials increased the accuracy and reduced the level of uncertainty. |
| Trends of the error bars and low uncertainty | The small trend lines and no overlapping suggest a high data accuracy level. |
| Method of the approach used. | The methods used in the whole investigation assisted in the determination of changes between the independent and dependent variables. This led to the establishment of the relationship between the two measures. |
| The range of temperature of the acetylsalicylic solution was selected. | The temperature ranged from 0 to 100 degrees which increased the accuracy of the investigation. |
| Use of excel software | The use of excel software enhanced the accuracy of the determined reaction rate. |
The followings are the sources and weaknesses that might have occurred during the experiments.
Table 6: Sources of errors and weakness
| Source of Error | Impact of the Error | Future Improvement |
| Loss of Heat
(Random error) |
Heat might have been lost during the heating of the solution, which might have reduced the accuracy of the rate of reaction recorded. | Double-check the temperature readings before recording the final results to increase accuracy. |
| Rounding off Errors
(Systematic error) |
The rounding off of the values of trials might have reduced the accuracy of the investigation. | Ensure to use the same significant figures in the whole investigation |
| Stirring the water
(Random error) |
Failure to stir the reactants of the water and aspirin solution might have reduced the accuracy of the rate of reaction recorded. | Ensure to stir the reactants before recording the time consumed to ensure heat is evenly distributed within the solution |
| Use of the beaker
(Random error) |
The use of a beaker might have resulted in contamination of the solution, which might have raised the boiling point of the solution and lowered its melting point. | One can adopt the use of a thermostatic |
| Waiting 25 seconds before recording
(systematic error) |
This might have reduced the accuracy of the rate of hydrolysis and temperature recorded. | Minimize the time interval to around zero seconds to enhance the accuracy of the data recorded |
The errors and limitations above in table 4 might have lowered the accuracy of the investigation and resulted in an increased percentage of uncertainty. Therefore, the finding and analysis of the data collected were able to support the hypothesis of the investigation. Hence, an increment in the temperature of reactants causes an increment in the overall rate of hydrolysis.
Extension and further experiments
This investigation can further be extended by checking the relationship between different temperatures and the rate of hydrolysis of other drugs, such as Tylenol. Tylenol is an alternative drug to aspirin which is widely used to reduce fever and relieve pain. One could also test other factors that affect the rate of hydrolysis, such as concentration and catalysts on different types of acids. An introduction of a catalyst in the reaction of hydrolysis actually speeds up the rate of hydrolysis, and the same also applies in increments of concentration. An increase in the concentration of one of the reactants causes an increase in the collisions of the molecules, which increases temperature that speeds up the rate of hydrolysis.
References
Trinh, H. B., Kim, S., & Lee, J. (2022). Recovery of rare earth elements from coal fly ash using enrichment by sodium hydroxide leaching and dissolution by hydrochloric acid. Geosystem Engineering, 25(1-2), 53-62.
Mishra, S. P. (2022). Phenolphthalein Indicator in Titrimetric Estimation of Benzoic Acid Solubility and Distribution in Water and Benzene-Buffer Solutions. Asian Journal of Chemical Sciences, 11(4), 1-7.
Wilatika, R. A. S. A., & Yonata, B. (2022). Implementation of a guided inquiry learning model to exercise students’ critical thinking skills on reaction rate material. Jurnal Pijar Mipa, 17(1), 34-40.
Shah, S. A. A., Ahammad, N. A., Din, E. M. T. E., Gamaoun, F., Awan, A. U., & Ali, B. (2022). Bio-convection effects on Prandtl hybrid nanofluid flow with chemical reaction and motile microorganism over a stretching sheet. Nanomaterials, 12(13), 2174.
Pornchai, I., Jing, L., Fei, Z. M., Yuan, Y. S., Farooq, M. U., & Kanjana, N. (2022). A review of chemical modification by using sodium hydroxide (NaOH) to investigate the mechanical properties of sisal, coir, and hemp fiber-reinforced concrete composites. Journal of Natural Fibers, 19(13), 5133-5151.
Wang, Y., Miao, J., Saleem, M., Yang, Y., & Zhang, Q. (2022). Enhanced adsorptive removal of carbendazim from water by FeCl3-modified corn straw biochar as compared with pristine, HCl, and NaOH modification. Journal of Environmental Chemical Engineering, 10(1), 107024.
 write
write