Abstract
Individuals with familial hypercholesterolemia, particularly homozygotes with the illness that presents through increased levels of LDL-C, present specific problems during pregnancy. With the help of this review, a complex relation between FH and pregnancy is explored, which also reveals that a specialized multidisciplinary approach should be employed to treat increased cardiovascular risks linked with maternal-fetal health when associated with HoFH in processes. The worldwide health burden of HoFH underdiagnosis and undertreatment is currently substantial, which requires more awareness campaigns, genetic screening activities, as well better access to diagnostic tools. The genetic background and diagnostic methods of the disease are discussed in relation to cascade screening while shedding light on new developments in genetic testing techniques, such as next-generation sequencing. These diagnostics techniques, including the FH Dutch Lipid Clinic Criteria and Simon Broom Method, are discussed regarding the diagnosis of HoFH based on different characteristics. Notably, phenotypic methods have limits, pointing to the urgency of common genetic testing tools. HoFH management related to pregnancy needs a reasonable balance between fetal safety and maternal cholesterol control. It is essential to stop the administration of statin medicine, and lipoprotein apheresis looks like an appropriate substitute. Comprehensive patient care is coordinated by genetic counselors, cardiologists, lipidologists, and obstetricians. The management of HoFH in pregnancy requires further research efforts, enhancement of public knowledge, and worldwide coordination. By focusing on these regions, we can make significant progress in diagnostics and develop efficient management plans that organize approaches for improving results among pregnant women with homozygosclerosis.
Introduction
FH is a genetic lipid disorder characterized by high LDL-C levels, which increases the risk of cardiovascular disease at an early age (1). A major symptom of homozygous familial hypercholesterolemia (HoFH), inherited from defective genes, constitutes a severe case of this hereditary disorder (2). The most characteristic finding of the FH is a marked 4-fold elevation of LDL-C associated with corneal arcus, some tendon xanthomas, and particularly atherosclerosis predisposition. Pregnancy is a high-risk condition for those with FH requiring specialized care. The complicated interrelation between the elevated cardiovascular risks associated with FH and physiological changes that take place during pregnancy points to the necessity of individualized, multidisciplinary counseling. The suggested possible complications of cardiovascular diseases and the effect on the health of the mother and fetus make such treatment of the FH during pregnancy important.
This review will discuss the diagnostic procedures, incidence rates, epidemiology information, and current treatment methods to provide a broad overview of FH during pregnancy. HoFH remains underdiagnosed and undertreated globally, which leads to increased cardiovascular morbidity (3). The low level of knowledge and acceptance of this disease also supports the need for a modified study that considers recent findings and progressive trends. This review seeks to fill in between current procedures and responsiveness upheaval led by managing hereditary hypertension during pregnancy. This review deals with the existing literature findings and includes recent epidemiological data to ensure a useful tool for academics, physicians, and health care practitioners; because of the multifaceted Factors, it is essential to have a profound knowledge of FHs in general and more so during pregnancy, where maternal well-being and fetal growth are maintained.
The prevalence of HoFH and its epidemiology
The condition termed familial hypercholesterolemia (FH), particularly the homozygous form (HoFH), is a global health threat from inherited mutant genes compound to high levels of LDL-C (4). The prevalence of HoFH varies worldwide, and its management requires an understanding of epidemiological settings. Consequently, consanguinity rates play a major role in determining the prevalence of HoFH, particularly where close relatives are encouraged to get into marriage. However, this is not the case for only a few other regions cannot have similar levels of HoFH prevalence in their own families as there are so many raised consanguinity within Middle Eastern countries. For instance, there is a higher incidence of genetic diseases like HoFH in Saudi Arabia and other countries that have consanguinity marriages (5). In Oman, the rate of consanguinity shapes family intermarriage and leads to a low detection of HoFH.
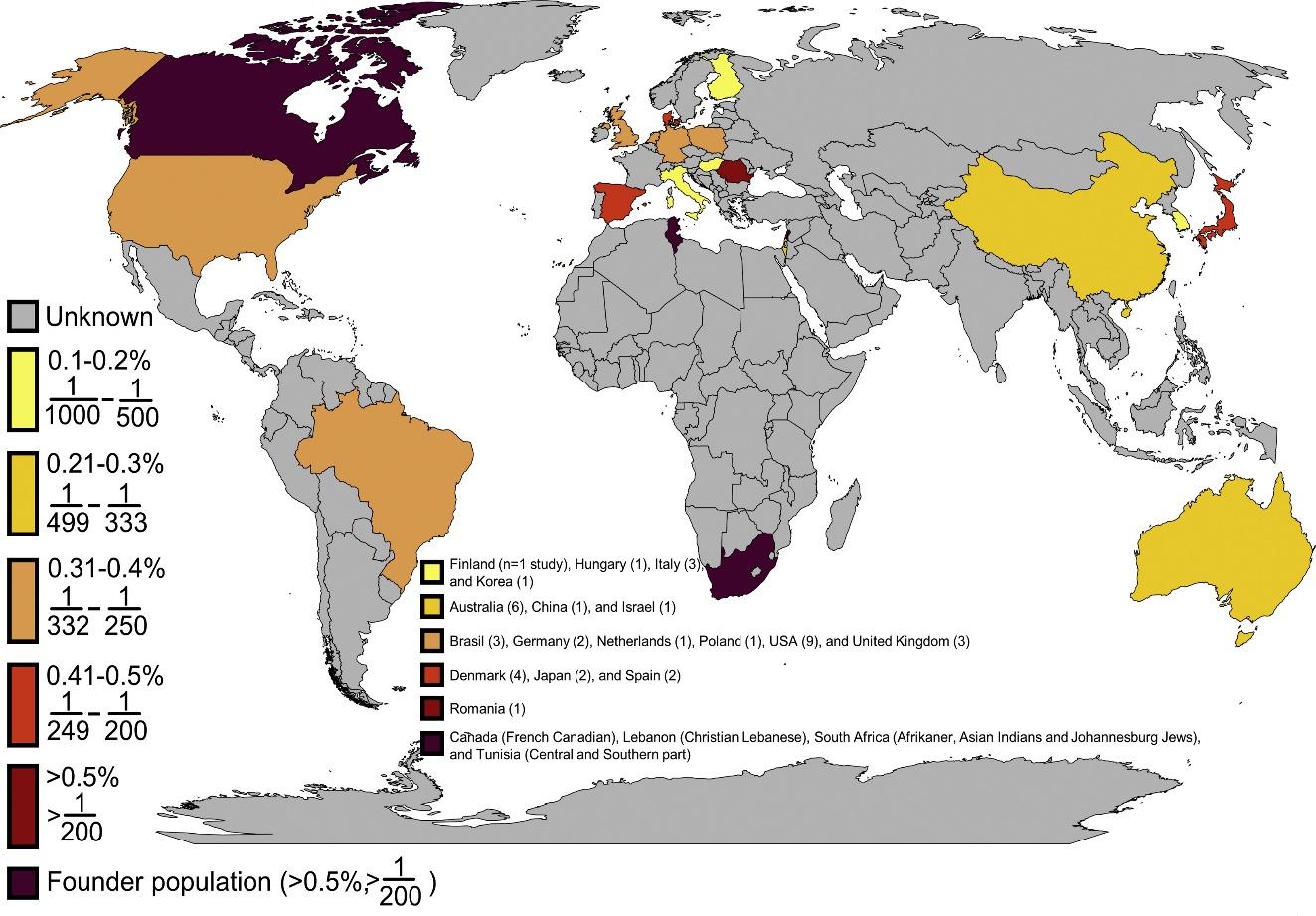
Source: https://www.sciencedirect.com/science/article/pii/S0735109720347501
Figure 1
FH prevalence is known to exist in 17 (9% of 195 nations), with four more countries reporting founder populations. This means that the prevalence of FH is unknown in 178 countries (91%). There are large data gaps in Africa, South America, and Asia due to the studies’ concentration in Europe, North America, East Asia, and Australia.
The latest data shows the global incidence of this condition, which is good for clarifying an update on HoFH prevalence and regional differences. The prevalence of HoFH in the Gulf region is estimated to be 1: 232, indicating a high prevalence in this field (6). In addition, it has been reported that the incidence in Dutch populations may range from one person per 160,000 (7). Based on hospitalization and patient registries, Denmark’s estimated definite and probable HoFH prevalence is 1 137 (8).
Undiagnosis and undertreatment of HoFH have a significant effect and can have dire consequences for the individuals affected. Underdiagnosis is due to several reasons, including its rarity and the lack of national registries in most countries (9). In Japan and the Middle East, many cases of HoFH are not diagnosed due to underdiagnosis. This may lead to suboptimal treatment and higher cardiovascular disease risks. It is estimated that about 14 – 34 million people worldwide suffer from FH, including HoFH; however, one percent has been diagnosed.
To fight the underdiagnoses and undertreatment of HoFH, a focused effort must be made to raise awareness, develop genetic screening programs, and create diagnostic resources that are accessible (10). Ethnicity and consanguinity rates’ impact on prevalence underlines the need for targeted screening in regions where such practice is common. To reduce the burden associated with HoFH and improve FH outcomes for patients, an integrated approach to FH management that includes cultural, genetic, and geographic factors is required. The global spread of HoFH is better understood, and early diagnosis in combination with personalized treatments has become increasingly important.
The Genetic Foundation and Diagnostic Methodologies
The effective diagnosis and cure of HoFH depend on the knowledge about its genetic background. The mutation in the genes linked to cholesterol metabolism results in HoFH, and among them, the family of LDLR, PCSK9Apo B100and (and LDLapl RAP) are the most popular. The LDLR gene is associated with a high percentage of HoFH cases in genetics because loss-of-function mutations disrupt the capacity for removal by receptor action against each particle of Low-Density Lipoprotein (11). Genetic testing method developments have significantly increased the accuracy and efficiency of detecting these mutations. Next-generation sequencing technologies, including whole exome sequencing or genetic panel approach, enable comprehensive analyses for multiple genes simultaneously; therefore, causative mutations could be more accurately detected.
The search for mutations associated with FH in high-risk families is conducted by cascade screening. This method allows identifying other family members affected using the test for a person’s first-degree relatives after FH confirmation. Cascade screening helps reduce the impact of FH-related cardiovascular risks regarding effective therapeutic therapies and preventive measures (12). However, the first thing that needs to be identified for cascade screening results to be required is an index case at the outset highlighting a focus on raising awareness and improving diagnosis rates.
Where genetic testing is not readily accessible and available, phenotypic diagnosis methods such as FH Dutch Lipid Clinic Criteria and the Simon Broom Method are used (13). Important features of the Simon Broom Method include clinical and metabolic characteristics such as high LDL-C levels, corneal arcus, and tendon xanthomas. However, this method has some drawbacks since additional diagnostic techniques may be necessary, as there could be an overlap between specific LDL-C levels that suggest HoFH and other hypercholesterolemia.
While phenotypic diagnosis provides an initial assessment, genetic testing remains definitive in confirming homozygosity. Apart from assisting in identifying the mutations, it also simplifies the identification of affected relatives through cascade screening. Despite such effectiveness, many issues still need to be addressed, particularly in regions with minimal genetic testing resources, underscoring the global need.
Characteristics and Phenotypic Identification
Where genetic testing is not readily accessible and available, phenotypic diagnosis methods such as FH Dutch Lipid Clinic Criteria and the Simon Broom Method are used. Important features of the Simon Broom Method include clinical and metabolic characteristics such as high LDL-C levels, corneal arcus, and tendon xanthomas (14). However, this method has some drawbacks since additional diagnostic techniques may be necessary as there could be an overlap between specific LDL-C levels that suggest HoFH and other hypercholesterolemia
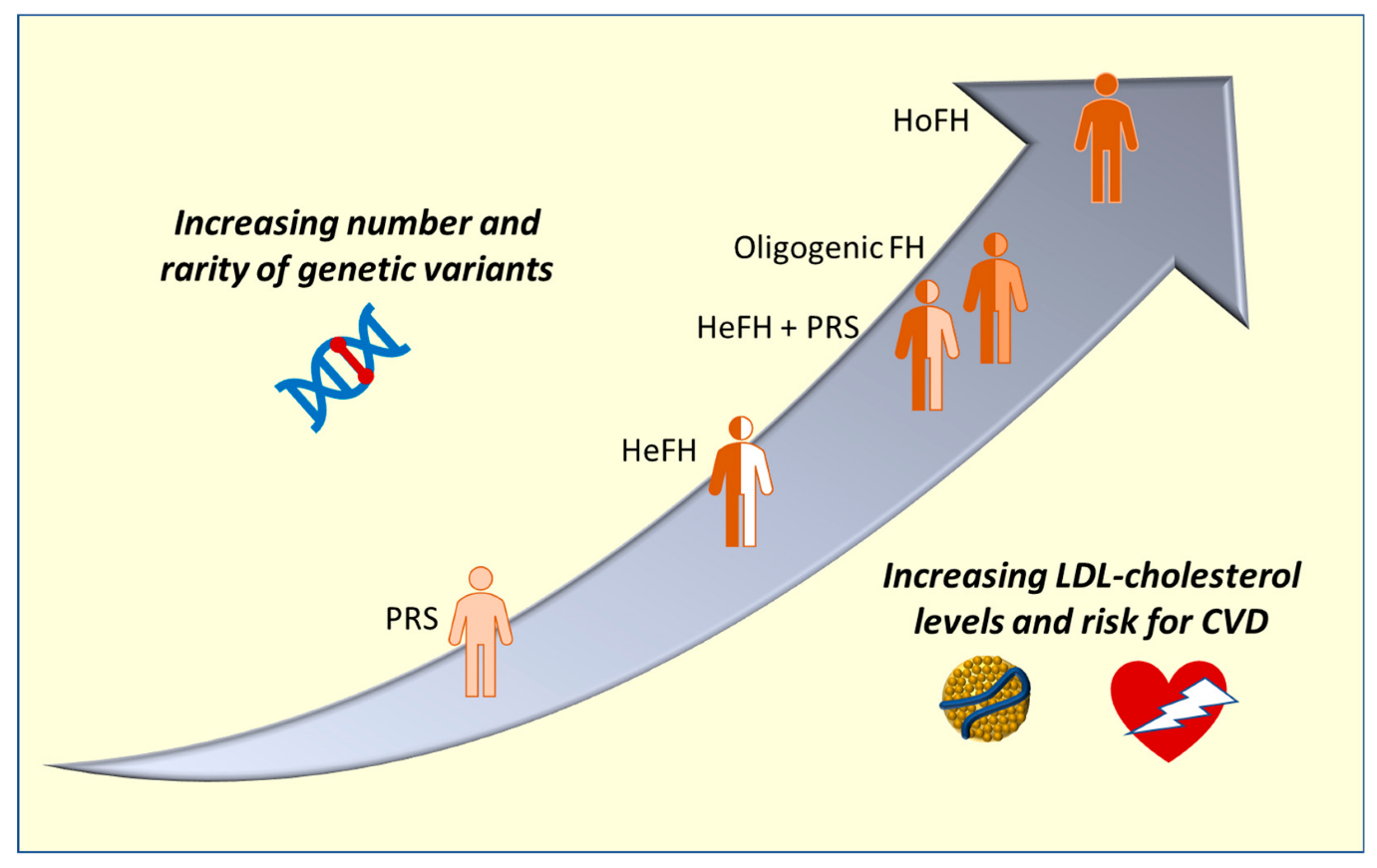
Source https://doi.org/10.3390/ijms24043224
Figure 2:
Variations in genetic status underlying hypercholesterolemia and their correlation with phenotypic. The x-axis shows the rise in LDL cholesterol and the corresponding risk of CVD, while the y-axis shows the quantity of uncommon genetic variations. HeFH stands for heterozygous familial hypercholesterolemia (FH), HoFH for homozygous FH, and CVD for cardiovascular disease. PRS stands for polygenic risk score.
While phenotypic diagnosis provides an initial assessment, genetic testing remains definitive in confirming homozygosity. Apart from assisting in identifying the mutations, it also simplifies the identification of affected relatives through cascade screening. Despite such effectiveness, many issues still emerge, particularly in regions with minimal genetic testing resources, underscoring the need for global.
The second phenotypic diagnostic method, which provides a comprehensive basis for FH identification, including that of HoFH patients, is the Dutch Lipid Clinic Criteria for FH (DLC) (15). This means that clinical criteria such as lipid profile tests, physical examination findings, and family history are built into this approach. These factors are quantified, and FH is estimated as a cumulative point score based on these parameters. The scoring system is greatly influenced by tendon xanthomas found for HoFH, which supports the importance of clinical awareness in procession diagnostics.
However, phenotypic diagnostic methods are inherently restricted. However, using specific cholesterol levels in the Simon Broom Method and the overlap between hypercholesterolemia can lead to misdiagnosis. Phenotypic approaches will not capture all cases, especially aberrant phenotype presentation or lack of xanthomas. Additionally, the subjective nature of clinical assessments may lead to unpredictability during diagnosis.
Techniques for Managing HoFH during Pregnancy
However, managing HOFH during pregnancy presents a myriad of intricate complications. Therefore, it requires an all-encompassing strategy that is attentive to the mother’s health and fetal development. Pregnancy creates an additional level of complication that further elevates the cardiovascular risks inherent in HoFH. A comprehensive and personalized solution is thus required to find a balance between regulating cholesterol levels in the mother, which could prove unsafe for her fetus. Of special importance to the management of FH is the discontinuation of statin therapy before conception and during pregnancy (16). Given that statins can have teratogenic effects on the fetus, cholesterol-lowering medications should not be used. Nevertheless, an abrupt stop leads to a worsening of cardiovascular problems for the mother and means that substitute treatments should be sought.
A successful alternative is lipoprotein apheresis, particularly if more traditional medications are either unavailable or contraindicated. This extracorporeal method provides a pleasant solution that removes the LDL cholesterol directly from the circulation, making it safe for both mother and fetus (17). However, despite the highly resource-intensive nature of lipoprotein apheresis despite its efficacy, access remains an ongoing issue. The management of HoFH during pregnancy requires complex and integrated strategies. Therefore, for full management of this multi-dimensional genetic disorder, it is highly essential that statin therapy be stopped and lipoprotein apheresis explored as a possible replacement. To ensure the best possible outcomes for the mother and child, teamwork among health professionals is becoming increasingly important as our knowledge of HoFH progresses.
Effective approaches are critical when managing homozygous familial hypercholesterolemia (HoFH) during pregnancy. The illustration provides insights into rare genetic variations and their relationship to LDL-cholesterol levels and cardiovascular disease risk by illustrating the correlation between distinct hereditary statuses in hypercholesterolemia. It is essential to comprehend the genetic background, as explained in the preceding text, to diagnose and treat HoFH (18) accurately. The graphic emphasizes the delicate balance needed to manage hypertrophic hypoxic syndrome (HoFH) during the distinct physiological state of pregnancy, complementing discussions on genetic alterations linked to the condition and highlighting developments in genetic testing techniques.
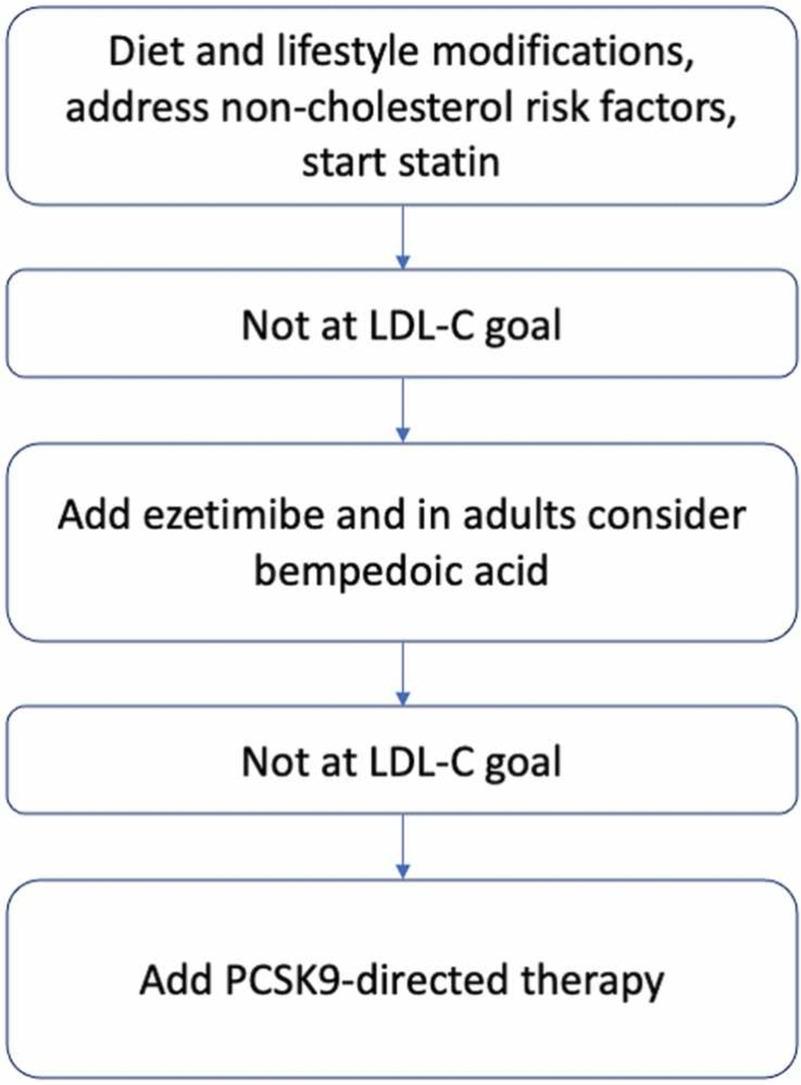
Source: https://doi.org/10.1016/j.phrs.2023.106857
Figure 3
The figure illustrates Familial hypercholesterolemia (FH) is an autosomal condition characterized by raised LDL-C and increased risk of ASCVD. A significant number of FH patients fail to meet prescribed LDL-C objectives even with maximal statin treatment. Alternatives that show promise include PCSK9 and ANGPTL3 targeting emerging treatments. The review examines current and future approaches, highlighting the significance of ongoing clinical studies for the therapy of FH.
Adopting a cross-cutting strategy involving genetic counselors, lipidologists, cardiologists, and obstetricians is crucial; This collaborative approach assures continuous monitoring, informed decision-making, and a full patient condition assessment. Critical components are periodic cardiovascular health assessments, fetal development, and cholesterol levels. Thus, family planning options and cascade screening are assisted by genetic counselors while obstetricians manage pregnancies and potential complications associated with them; lipidologists specialize in personalizing treatment modalities, etc.
Conclusion
In conclusion, the management of HoFH in pregnancy can be considered a challenging issue requiring teamwork between different medical specialists and appropriate planning regarding treatment methods. To competently sail through the challenges posed by this genetic condition, it is essential to halt statin therapy and look for other treatment options, such as lipoprotein apheresis, in addition to structural collaboration amongst cardiologists. This research underscores the need for further studies and greater recognition, noting that HBH during pregnancy continues to require sustained efforts aimed at a better understanding of this condition. The challenges that are mentioned above point to the need for innovative solutions, accessible diagnostics as well as international cooperation on behalf of medical specialists. As we learn more, continuous projects of ongoing research and continued public awareness endeavors are fundamental to ensure that management practices improve and, ultimately, outcomes for mothers with their children will be enhanced. By prioritizing scientific research and awareness, we create opportunities for improved clinical practices and the quality of life that people suffering from HoFH have to go through during their pregnancy.
References
- Anagnostis P, Antza C, Florentin M, Kotsis V. Familial hypercholesterolemia and its manifestations: Practical considerations for general practitioners. Polish Heart Journal/Kardiologia Polska. 2023 Nov 1;81(11).
- Tromp TR, Hartgers ML, Hovingh GK, Vallejo-Vaz AJ, Ray KK, Soran H, Freiberger T, Bertolini S, Harada-Shiba M, Blom DJ, Raal FJ. Homozygous Familial Hypercholesterolaemia–Worldwide Experience. Lancet (London, England). 2022 Feb 2;399(10326):719.
- Cuchel M, Raal FJ, Hegele RA, Al-Rasadi K, Arca M, Averna M, Bruckert E, Freiberger T, Gaudet D, Harada-Shiba M, Hudgins LC. 2023 Update on European Atherosclerosis Society Consensus Statement on Homozygous Familial Hypercholesterolaemia: new treatments and clinical guidance. European Heart Journal. 2023 May 2:ehad197.
- Abifadel M, Boileau C. Genetic and molecular architecture of familial hypercholesterolemia. Journal of Internal Medicine. 2023 Feb;293(2):144-65.
- Batran AH. Detection of Novel Mutation Genes in Familial Hypercholesterolemia (FH) Disorder in Saudi Arabia (Doctoral dissertation, King Abdulaziz University Jeddah-Saudi Arabia).
- Mahzari M, Zarif H. Homozygous familial hypercholesterolemia (HoFH) in Saudi Arabia and two cases of lomitapide use in a real-world setting. Advances in Therapy. 2021 May;38:2159-69.
- de Sá AC, Gomes CS, Prates EJ, Brant LC, Malta DC. Prevalence and factors associated with possible cases of familial hypercholesterolemia in Brazilian adults: a cross-sectional study. Scientific Reports. 2023 Nov 22;13(1):20459.
- Benn M, Watts GF, Tybjaerg-Hansen A, Nordestgaard BG. Familial hypercholesterolemia in the Danish general population: prevalence, coronary artery disease, and cholesterol-lowering medication. The Journal of Clinical Endocrinology & Metabolism. 2012 Nov 1;97(11):3956-64.
- Tromp TR, Hartgers ML, Hovingh GK, Vallejo-Vaz AJ, Ray KK, Soran H, Freiberger T, Bertolini S, Harada-Shiba M, Blom DJ, Raal FJ. Worldwide experience of homozygous familial hypercholesterolemia: retrospective cohort study. The Lancet. 2022 Feb 19;399(10326):719-28.
- Heather DM, Zierhuta A. Development of an Implementation Framework for Overcoming Underdiagnoses of Familial Hypercholesterolemia in the USA.
- Bajaj A, Cuchel M. Advancements in the treatment of homozygous familial hypercholesterolemia. Journal of Atherosclerosis and Thrombosis. 2022 Aug 1;29(8):1125-35.
- Lee CJ, Yoon M, Kang HJ, Kim BJ, Choi SH, Jeong IK, Lee SH. 2022 Consensus statement on the management of familial hypercholesterolemia in Korea. The Korean Journal of Internal Medicine. 2022 Sep;37(5):931.
- Hedegaard BS. Familial Hypercholesterolaemia: Detection, diagnostic issues and collaboration between lipid clinics and general practice.
- Timoshchenko O, Ivanoshchuk D, Semaev S, Orlov P, Zorina V, Shakhtshneider E. Diagnosis of Familial Hypercholesterolemia in Children and Young Adults. International Journal of Molecular Sciences. 2023 Dec 25;25(1):314.
- Bucholz EM, Rodday AM, Kolor K, Khoury MJ, de Ferranti SD. Prevalence and predictors of cholesterol screening, awareness, and statin treatment among US adults with familial hypercholesterolemia or other forms of severe dyslipidemia (1999–2014). Circulation. 2018 May 22;137(21):2218-30.v
- Lan NS, Bajaj A, Watts GF, Cuchel M. Recent advances in managing and implementing care for familial hypercholesterolemia. Pharmacological Research. 2023 Jul 17:106857.
- Safarova MS, Moriarty PM. Lipoprotein Apheresis: Current Recommendations for Treating Familial Hypercholesterolemia and Elevated Lipoprotein (a). Current Atherosclerosis Reports. 2023 Jun 5:1-4.
- Lan NS, Bajaj A, Watts GF, Cuchel M. Recent advances in managing and implementing care for familial hypercholesterolemia. Pharmacological Research. 2023 Jul 17:106857.
 write
write