The wound is one of the significant clinical burdens that is affecting patients worldwide. To deal with these burdens, it is essential to understand the physiologies in wound care and healing (Velnar, Bailey, & Smrkolj 2009). Wound healing is a complex process that involves a collaboration of various cell types, vascular systems, mediators, and cytokines (Wallace Basehore & Zito, 2017 ). Researching wound healing can provide a clear understanding of the general cellular and molecular processes involved in the healing of wounds. A range of effective therapeutic strategies for wound care and healing can be developed through research on wound healing. Clinical needs for patients with wounds can be identified to improve the outcomes of patients with chronic wounds. At the end of this research, the wound healing mechanisms and the factors that predict the healing process will be identified.
Additionally, a clear understanding of wound healing therapies will be provided. This study will avail research-based interventions for wound complications which will help clinicians optimize the provision of satisfying customized care for patients with wound problems. Healthcare providers can use study findings in drafting new therapies that can effectively improve patients’ outcomes to ensure that wound care programs are inclusive despite the unique needs of various patients. These inclusive personalized, and effective healthcare services will ensure that the healthcare goal of providing effective healthcare to all is attained.
Physiology of wound healing
Wound healing involves regeneration and repair processes as a physiological response to injury. Wound regeneration occurs in tissues with sufficient cell populations that can facilitate mitosis, such as the epithelium (Theoret, 2016). This process involves the replacement of damaged tissues with new tissues structurally and functionally similar to the lost tissues. Wound repair replaces damaged tissues with undifferentiated scar tissues (Theoret, 2016). Wound healing processes triggered by tissue injuries can be explained into four phases: coagulation and hemostasis, inflammation, proliferation, and remodeling (Velnar, Bailey& Smrkolj 2009).
Phases of wound healing
Coagulation and Hemostasis
This Is the first stage of the wound-healing process. It occurs instantly after injury and completes within hours (Theoret, 2016). This mechanism targets to protects the vascular system and ensures that other body tissue’s functionalism is unharmed despite an injury. This mechanism also provides a matrix essential for the invasion of cells needed in the later stages of healing (Velnar, Bailey& Smrkolj 2009).
This phase of wound healing is characterized by a response in which the damaged endothelial cell membrane produces phospholipids which transform into a type of fatty acid known as arachidonic acid and their metabolites that aid in the reduction of blood flow to the injured tissues (Theoret, 2016). This process starves oxygen and nutrients to the surrounding tissues and increases glycolysis, decreasing Ph that contributes to platelet activation. The activated platelets activate the extrinsic and intrinsic pathways, resulting in blood clotting on the injured layer to prevent further bleeding (Grubbs & Manna, 2018).
The clotting forms an extracellular matrix with fibrinous, vitronectins, thrombospondins, and fibronectins, filling the wound and providing a scaffold for migrating cells. The clots and platelets filling the wound site contain α-granules with cytokinin and growth factors, which promote healing by activating and attracting neutrophils, macrophages, fibroblasts, and endothelial cells (Velnar, Bailey& Smrkolj 2009). The platelets contain the vasoactive amines that modify the permeabilities and vascular tones of the injured cells to increase and create an environment that will facilitate the supply and diffusion of nutrients and oxygen to the new arriving cells (Theoret, 2016).
Inflammation
This wound-healing phase aims to create an immune barrier against wound invasion with microorganisms. The first phase, which is the early inflammatory phase, is characterized by neutrophil functionalities that destroy the bacteria or any foreign material within the wound sites through a process known as phagocytosis (Wallace, Basehore, & Zito,2017 ). Neutrophils’ attraction to the wound tissues occurs within 24 hours to 36 hours of injury, and these attractions are aided by attractive chemo agents such as transforming growth factor-β (TGF- β), formyl methionyl peptides, C3a and C5a that are produced by platelets products and bacteria (McCance, & Huether, 2018). Through margination, neutrophils get sticky and begin adhering to endothelial cells of capillary venules surrounding the wound (Velnar, Bailey& Smrkolj 2009). The neutrophils are rolled along the endothelium surface by the blood flow push. The endothelial cells then secrete Chemokines, rapidly activating a stronger adhesion system mediated by integrins on the cell surface. Rolling and migration of the cells are ceased, and they move out of the venules and squeeze between the endothelial cells through a process termed diapedesis (Velnar, Bailey& Smrkolj 2009). The neutrophils within the tissues die and are then phagocytized by wound macrophages or fibroblasts. This is when (Theoret, 2016) suggests we can say the first inflammatory phase is complete.
The late inflammatory phase occurs within 48 to 72 hours after sustained injuries. In this stage, macrophages appear at the wound sites and continue phagocytosis. Macrophages are cells that have undergone phenotypic changes upon arrival to the injured tissue. These macrophages release products for Collagen and elastin breakdown. They also release cytokines like (TGF-β, PDGF, leukotriene B4, ) and platelet factors (Velnar, Bailey& Smrkolj 2009). These products aid them in the proliferation and movement of Fibroblast and endothermal cells that are essential in repairing damaged tissues and forming their blood vessels(Angiogenesis). After 72 hours, lymphocytes are attracted to the injured zone through the action of interleukin-1 (IL-1), immunoglobulin breakdown products, and complement products. interleukin-1 regulates the production and activity of the enzyme collagenase required in modeling and generating the extracellular matrix.
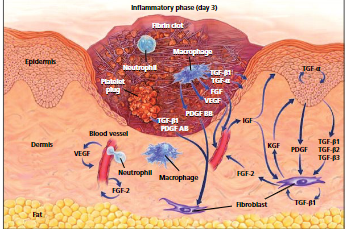
Figure 1: Molecular and cellular components in a wound Day 3. (Source, Theoret, 2016)
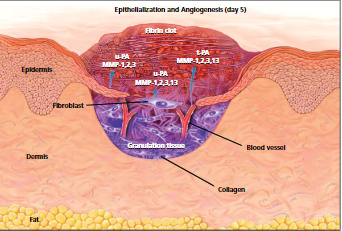
Figure 2: Epithelization and angiogenesis. (Source, Theoret, 2016)
Proliferative Phase
This phase of wound healing starts on the third day of wound healing and lasts up to 2 weeks. During this phase, the macrophage is invaded to dissolve the clotting tissue, and Fibroblasts, connective tissue, and epithelial and endothelial cells rapidly occupy the wound, preparing to cover the wound (Theoret, 2016). The macrophages secrete biochemical mediators such as Transforming Growth Factor-beta (TGF-β), angiogenesis factors, and matrix metalloproteins responsible for wound healing. The (TGF-β), stimulates the fibroblasts that enter the wound lesions to synthesize and produce collagen precursor procollagen. Angiogenesis factors stimulate the formation of capillary buds in the endothelial cells that will grow in the lesion. On the other hand, Matrix metalloproteinase helps remodel Collagen and fibrin, which are the extracellular matrix proteins (McCance & Huether, 2018).
Wound surrounding connective tissues rich in invasive cells, new capillaries, and lymphatic vessels facilitate the growth of granulation tissues, making a wound appear reddish. Capillary buds from vascular endothelial cells later grow within the wound zone. The capillaries differentiate to form larger vessels during the wound repairs, and as the process continues influx of nutrients and removal of wastes within the wound zone is promoted. New lymphatic vessels are also formed in the tissues using the mechanism above. For wound protection purposes, the migrating epithelial cells from both sides of the wound start to seal the wound. The migration of epithelial cells under the clot is aided by MMPs that unravel Collagen. Collagen undergoes a series of matrices to assemble a final collagen matrix within months. The fibroblasts help in secreting the Collagen and connective tissue proteins essential in wound healing. After the final collagen matrix of the wound is formed, the wound contracts, and normally an inward movement of the edges of the wound is noticed (McCance & Huether, 2018).
Remodeling phase and maturation
This is the final stage of wound healing, responsible for forming the final scar tissues and their epithelium layer (Velnar, Bailey& Smrkolj 2009). Tissue remodeling begins after several weeks of the injury and can last up to even 2 years (McCance & Huether, 2018). This phase records a series of events like the differentiation of cells and the formation and remodeling of the scar. Tissue regeneration and contraction of the wound continue in this final stage. As the wound heals, densities of macrophages and fibroblasts within the wound reduce, and over time the blood flow and metabolic activities within the injured site decrease. The tensile power of the wound gradually increases with collagen collection on the wound site. Finally, a fully matured scar comprised of fewer cells and blood vessels with an improved tensile strength is formed. Graph 1 below provides a sequential illustration of the stages involved in wound tissue repair.
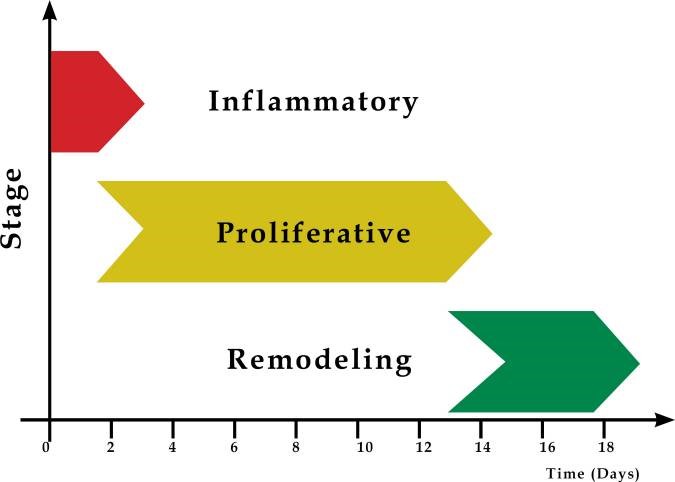
Graph 1: Stages of wound tissues repair
Pathophysiology
Wound healing leads to the formation of hypertrophic and keloid scars. Hypertrophic scarring usually occurs four to eight weeks after a wound infection and is characterized by wound closure with excess tension or traumatic skin injury; they are normally flat scars. Keloids appear firm. Tender blossomed tumors that are shiny and, at times, telangiectasia. They appear pinkish or purplish, with the borders of these tumors being well-demarcated but with irregular outlines (Grubbs & Manna,2018). Majorly hypertrophic scarring develops on wounds within the tensive anatomic locations like knees, ankles, shoulders, prosternum, and neck (Grubbs & Manna,2018). Keloid scars or lesions develop on tender zones like eyelids, cornea, genitalia, mucous membranes, and palms (Gauglitz et al.,2011). These two lesions are pruritic. However, keloids present with pains and hyperesthesia.
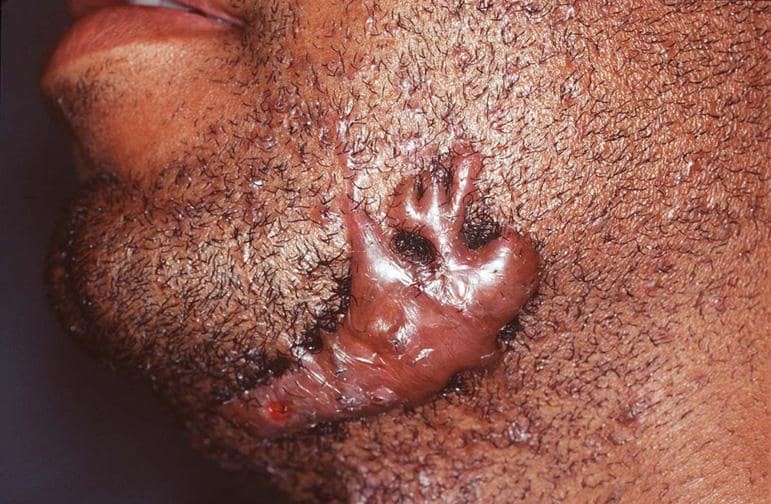
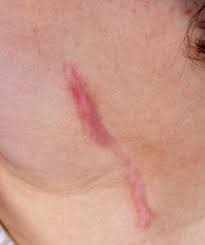
Figure 3: Keloid outlines source vs. hypertrophic scars (Berman,2022).
The formation mechanism of these scars’ formation is not well documented, but the overproduction of fibroblast proteins characterizes both scars. This explains that the normal production sequence of extracellular matrix proteins like collagen and fibronectin that aid in the repair of tissue alterations to cause overproduction of these proteins can cause this scar (Gauglitz et al.,2011). Additionally, this occurrence can be explained by the failures in the effective downregulation of wound-healing cells caused by genetic factors, infections, traumas, or even inflammations (Gauglitz et al.,2011). Graph 2 below shows the pathophysiology of excessive scar formation.
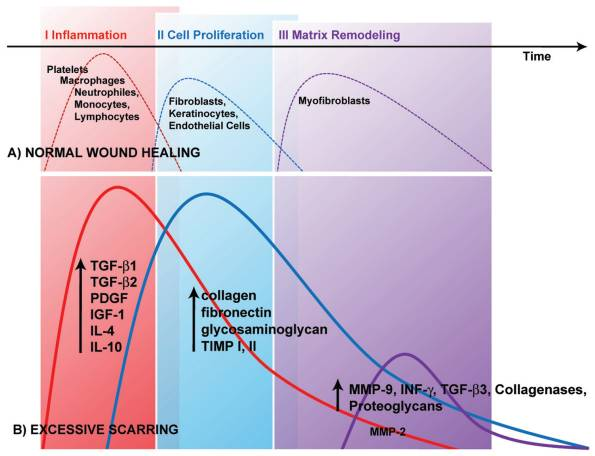
Graph 2: Scar formation. (Source Gauglitz et al.,2011).
The wound healing process follows the normal healing phases: inflammation as the first phase, proliferation as the second phase, and finally, remodeling. Platelet degranulation aids in the activation of s series of cytokines that serve as chemotactic agents that produce neutrophils, macrophages, fibroblasts, and epithelial cells (Gauglitz et al.,2011). Section (A) in the graph above shows the normal functioning of the chemotactic agents to attain a balance of new tissue biosynthesis and degradations mediated by remodeling of the extracellular matrix. Section (B) in the graph above shows situations with excessive scar formation. A challenge in the function of the wound healing regulatory mechanisms that oversee or aid the whole wound healing process leads to the excessive synthesis of collagens or defective remodeling matrix, and the inflammatory period can be prolonged. The wound-healing defective phases in (B) can explain the occurrence of keloids and hypertrophic scars (Gauglitz et al.,2011).
Impact of this research on patients and health care industry
This research on wound healing will have a series of outcomes for patients and the healthcare industry. These effects or outcomes can be of different perspectives, including clinical outcomes, emotional effects, and positive or negative impacts on patients and the health industry. Clinically this research will avail rich literature on the wound healing processes and various body mechanisms involved in wound healing. By reviewing various literature, potential wound healing therapies, technologies, and treatments shall be critically reviewed. Clinicians and patients can access updated information on the advances and innovations in wound healing technologies and treatments. By availing of relevant updated repository information on wound healing, clinicians can readily access repository information on improved research-based wound healing procedures. Adopting the newly availed ideas in their treatments for patients with wound complications can improve patient outcomes.
This research will also have emotional impacts on patients’ well-being. Patients with chronic wounds can be devastated by their condition, and they might fear that they can be culprits of stigmatization; thus, their confidence and self-esteem in social gatherings can deteriorate, and they even prefer to be isolated. These negative impacts that come with wound complications can dictate poor quality lives, and they are likely to be unsatisfied with their lives. By this research promoting improved patient outcomes through providing knowledge and evidence-based practices in wound healing, patients can be relieved of their burdens gradually. Those with chronic wounds can finally have hope of recovering their initial physical status. In the long term, when the penetration of newly researched wound healing practices is fully implemented, the overall; patient outcome can be improved. The clinicians can improve their client’s confidence, and the pains and suffering of patients and their social concerns can be mitigated. The overall result shall be the attainment of a more reliable wound-healing professional task force that will promote patients’ overall well-being and improve their quality of life.
The health sector shall be availed with information to improve their medications strategies to improve their services. By these sectors adopting the discussed strategies on wound care, their services can be reliable, and productivity in healthcare can record positive outcomes. The latest medications in wound healing can be attained at favorable costs and hospital stays that have cost implications through the adoption of recent competent wound healing and care medications. Healthcare care can be reduced. Additionally, healthcare wound care and healing educators can access updated knowledge that can be used in drafting modern wound healing technologies, therapies, and medications.
This research also has some negative impacts that are more affiliated with the health sector. This research can present information that can attract a conflict of interest regarding wound healing and care in the healthcare sector. The wound healing clinical gaps this research shall identify can trigger healthcare debates that likely trigger negative and positive influences.
Latest research in wound healing
The latest research technologies in wound healing use the regenerative wound healing mechanism. These technologies are centered on biomedical research, including bioactive wound dressing, gene therapies, stem cell therapies, targeted drugs, smart wound dressing, and skin bioengineering. Some of the emerging innovative wound healing techniques include;
Nanotherapeutic-based strategies
This technology research involves using various nanomaterials of antimicrobial peptides, antimicrobial agents, interferons, and growth factors directed to the wound to treat wounds. This nanotechnology is suggested to have a huge influence in regulating and controlling hemostasis and microbial infection and promoting the proliferation of cells, and it facilitates the delivery of drugs to deep tissues to ease the healing of chronic wounds (Kolimi et al., (2022)
Stem cell therapy
This strategy of wound healing research has reported that stem cell therapies promote the secretion of pro-regenerative cytokines and growth factors to promote the growth of skin regeneration when treating chronic wounds (Yamanaka, 2020), (Brown et al., 2019) & (Kolimi et al., (2022). This type of therapy is ideal for supporting natural skin healing by its ability to transform into any cell. Among the commonly utilized stem cells include Embryonic Stem Cell, Induced Pluripotent Stem Cells (iPSCs), and Mesenchymal Stem Cells (MSCs) (Kolimi et al., 2022)
Bioprinting-Based Strategies
This technology in wound healing provides the fabrication of compatible artificial skins through layers of deposition of cells, biomaterials, growth factors, and biomolecules. This skin bioprinting technique has been used to heal serious non-healing wounds like burn wounds, venous ulcers, diabetic foot ulcers, and pressure ulcers. Kolimi et al. (2022) further elaborate on the latest research on 3d bioprinting. Per their argument, to date, bioprinter skin equivalent containing all skin elements is still the subject of research.
Extracellular Matrix-Based Strategies
Chronic wounds stall their healing due to reduced ECMs due to high concentrations of fibroblasts, high metalloproteinase matrices, and lengthy inflammatory phases. Restoring these wounds with ECMs can facilitate the closure of these wounds. Researchers suggest that utilizing derived bio-engineered tissues is ideal for promoting wound healing Kalva (et al.,2021) & (Kim et al .,2020).
Platelet-Rich Plasma (PRP)-Based Strategies
This technique stimulates wound healing. The PRPs contain concentrations of platelets higher than those in the bloodstream and thus culture concentrations of growth factors that promote wound healing. The concentrated growth factors in the wound zones regenerate skin tissues; they act as signal molecules that influence cellular metabolisms. Reports suggest that the first PRP performance was clinically reported to be used in treating leg ulcers using collagen-embedded platelets. Later PRP injections and Gels are currently used in healing various wound etiologies (Kolimi et al., 2022).
Cold Atmospheric Plasma Therapies
Cold plasmas utilize biological and physical mechanisms for healing wounds. The biological mechanisms exploit the cellular processes that produce the DNA that damages the cell membranes of bacteria. The physical mechanism of wound healing via cold plasma is attained by the generation of free radicles and reactive oxygen species (ROS) or reactive nitrogen species (RNS) that promote wound healing (Kleineidam et al.,2019). Cold plasma treatments on wound assessments by various clinical research suggest that some outcomes include reduced bacterial concentration, rapid wound healing, and fastened migration of tissues within wound zones (Kolimi et al., 2022).
Application of this research
The vast knowledge of wound healing techniques can equip the researcher with an in-depth understanding of wound healing mechanisms and appropriate treatment plans for various chronic or normal wound types. These understandings will ensure that as a health care provider, I should optimize wound therapies ideas and easily identify wound complications approaching a complicated healing process. I can also identify potential gaps in wound healing that require further research.
Patients can be availed of wound treatment options that are aimed at improving their outcomes. Modern wound healing technologies can ensure patients receive customized wound care that considers all client-centered needs. Furthermore, healthcare professionals can gain insights into the advances in wound healing technologies and structure their healthcare to fit the trending wound healing guidelines, procedures, and options.
Conclusion
Wound is among the clinical burdens that affect a huge population across the globe. The wound healing process can be complicated, but its physiology can be broken down into four phases Coagulation and Hemostasis, inflammatory, proliferative, and remodeling. They can heal normally as wound healing undergoes these four phases or develop excessive scars. With the latest advancements in wound healing techniques, optimizing medications for wound healing can lead to improved patient outcomes and promote the attainment of the healthcare goal of quality health for all.
References
Brian Berman, M.D. (2022). Keloid and hypertrophic scar, Background, Pathophysiology, Etiology. Medscape. Available at: https://emedicine.medscape.com/article/1057599-overview (Accessed: April 2, 2023).
Brown, C., McKee, C., Bakshi, S., Walker, K., Hakman, E., Halassy, S., … & Chaudhry, G. R. (2019). Mesenchymal stem cells: Cell therapy and regeneration potential. Journal of tissue engineering and regenerative medicine, 13(9), 1738-1755.
Gauglitz, G. G., Korting, H. C., Pavicic, T., Ruzicka, T., & Jeschke, M. G. (2011). Hypertrophic scarring and keloids: pathomechanisms and current and emerging treatment strategies. Molecular medicine, 17(1), 113-125.
Grubbs, H., & Manna, B. (2018). Wound physiology.
Grubbs, H., & Manna, B. (2018). Wound physiology.
Kalva, S. N., Augustine, R., Al Mamun, A., Dalvi, Y. B., Vijay, N., & Hasan, A. (2021). Active agents loaded extracellular matrix mimetic electrospun membranes for wound healing applications—Journal of Drug Delivery Science and Technology, 63, 102500.
Kim, B. S., Das, S., Jang, J., & Cho, D. W. (2020). Decellularized extracellular matrix-based bioinks for engineering tissue-and organ-specific microenvironments. Chemical reviews, 120(19), 10608-10661.
Kleineidam, B., Nokhbehsaim, M., Deschner, J., & Wahl, G. (2019). Effect of cold plasma on periodontal wound healing—an in vitro study. Clinical oral investigations, 23, 1941-1950.
Kolimi, P., Narala, S., Nyavanandi, D., Youssef, A. A. A., & Dudhipala, N. (2022). Innovative Treatment Strategies to Accelerate Wound Healing: Trajectory and Recent Advancements. Cells, 11(15), 2439.
McCance, K. L., & Huether, S. E. (2018). Pathophysiology-E-book: the biologic basis for disease in adults and children. Elsevier Health Sciences.
Theoret, C. (2016). Physiology of wound healing. Equine wound management, 1–13.
Velnar, T., Bailey, T., & Smrkolj, V. (2009). The wound healing process: an overview of the cellular and molecular mechanisms. Journal of international medical research, 37(5), 1528-1542.
Velnar, T., Bailey, T., & Smrkolj, V. (2009). The wound healing process: an overview of the cellular and molecular mechanisms. Journal of international medical research, 37(5), 1528-1542.
Wallace, H. A., Basehore, B. M., & Zito, P. M. (2017). Wound healing phases.
Yamanaka, S. (2020). Pluripotent stem cell-based cell therapy—promise and challenges. Cell stem cell, 27(4), 523–531.
 write
write