Introduction
New product development is growing faster because of the pharmaceutical industry’s expansion. There are more innovative medications and pharmaceuticals being developed by researchers in biology nowadays (Gutierrez et al., 2020). This is the reason why a greater emphasis has been found on business strategy and marketing techniques in the pharmaceutical sector. AstraZeneca has come to the state where it is suffering to move forward. AstraZeneca has adopted that the more extraordinary approach be used in this investigation. However, AstraZeneca required the help of a consultancy so that it can remove the obstacles from its path and at least develop adequate strategies to fight its issues. Hence, the present report, it is aimed to present an adequate strategic analysis including where AstraZeneca is lagging and where it requires improvements.
Analysis of the Current Situation of AstraZeneca
AstraZeneca is known to be a global biopharmaceutical company that emphasizes the commercialization of medicines prescribed and the production of medicines. The products that are mainly offered are for the treatment of cardiovascular, respiratory, oncology, infection, neuroscience-related diseases, and others (www.astrazeneca.com, 2022). The company operates manufacturing around more than 40 drugs in the UK market. The products composed by AstraZeneca are Atacand HCT, Crestor, Lokelma, Seloken ZOK, XIGDOU XR. Among which certain drugs are most renowned and common involving BEVESPI AEROSPHERE, BREZTRI AEROSPHERE, BRILINTA Tablets, FASLODEX, IMFINZI, FluMist, LOKELMA, and others. The biggest competitors of AstraZeneca in the global market involves GSK, Bayer, Novartis, Bristol Myers Squibb, and Teva Pharmaceuticals.
Figure 1: Covid-19 vaccine manufactured by AstraZeneca
(Source: www.astrazeneca.com, 2022)
AstraZeneca mainly emphasizes the drugs that are meant for treating Type 2 diabetes and cancer. Another outstanding drug was Tagrisso gained much revenue in 2020. AstraZeneca offers great opportunities and better employment within the pharmaceutical industry. The company promises to provide an effective business policy and an efficient balance between work and diversity within the industry. As opined by Runiewicz-Wardyn and Eliashvili (2022), 95% of employees of AstraZeneca have considered the firm to be a great workplace as they can work with an effective policy leading to a growth of the production of drugs and thus initiating the overall revenue of the company.
The company also has the following opportunities in terms of its future growth,
- AstraZeneca holds opportunities in terms of expanding the patient base by making the medicines available to people in previously unreachable regions is made possible by gaining access to new markets.
- The company holds significant opportunities by making patient-centered approaches, fully integrated ecosystems when it comes to innovation. To better serve our patients in emerging markets, the company is focusing on efforts there as well.
- At AstraZeneca, the partnership is more than simply a term or a goal. Hene, there is a huge opportunity to work actively with local and central governments as well as local partners. Wherever possible, there are also significant opportunities to collaborate closely with nearby healthcare providers and financial institutions.
The drug release and implementation of vaccines all have been initiated by AstraZeneca, thus it provides ample future growth scopes in the field of medicine. Besides this, there are several drawbacks of the medicines manufactured by AstraZeneca which involves the Covid-19 vaccine which has been reported to have symptoms like headache, nausea, fever, muscle pain, and body ache. Recent studies on Covid-19 vaccines reveal that Vaxzevria which was manufactured by AstraZeneca evidences certain risks that include blood clotting followed by low blood platelet. Another major drawback of the vaccine is the eligibility criteria in terms of age which is above 18 years of age (www.astrazeneca.com, 2022).
Individuals have recently reported several allergic reactions to the vaccine manufactured by AstraZeneca. The symptoms include skin rashes, swelling of the body, and certain respiratory issues. There are several modes and strategies implemented to improve the performance of AstraZeneca including fragment-based lead generation. According to Montastrucet al. (2021), in this project, the changes are determined and it focuses on the framing of AstraZeneca fragments based on screening practices. The model includes optimization of the screening practices, determination of quality, and usage of ligand-related calculations, the FBLG is used for a detailed study on antibacterial drug delivery and enzymes.
Besides this, AstraZeneca confronts several challenges based on maintaining research and development at an optimum level within the pharmaceutical firms. As per the view of Freedman et al. (2021), the 5 R strategy is being implemented to get an overview of the quality and their performance which includes the right target, right patient, right safety, right tissue, and right commercial potential. AstraZeneca is currently implementing this strategy to determine the research and developmental status of the company and its overall productivity. With the increasing population, there is a higher demand for vaccines, thus to enhance the vaccine production the supply gets indulged with more than 20 suppliers. Chemistry, manufacturing, and controls related to the supply chain and the overall productivity, during this process of CMC the manufacturing procedure is repeated to ensure better yields and best quality products across the supply chain.
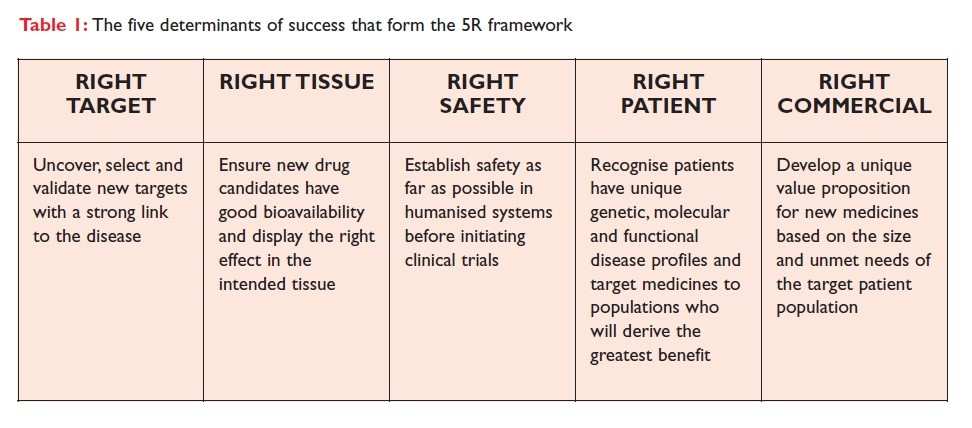
Figure 2: 5R strategy implemented by AstraZeneca to improve the productivity
(Source: www.astrazeneca.com, 2022)
Strategic Recommendations
Fragment-based lead generation (FBLG)
FBLG is now a valid “hit-finding method” at AZ (AstraZeneca) for all water-soluble targets. It is seen as a supplement or alternative to existing “hit-finding approaches”, like “HTS or encoded library screening”, as part of AZ’s lead-generating tools development (Holdgate et al., 2019)..
Although fragment campaigns typically target structurally enabled targets, the “devoted fragment chemistry team and strengthened biophysical capabilities” allow AZ to use an FBLG approach for high-interest targets (Kettle et al., 2020).
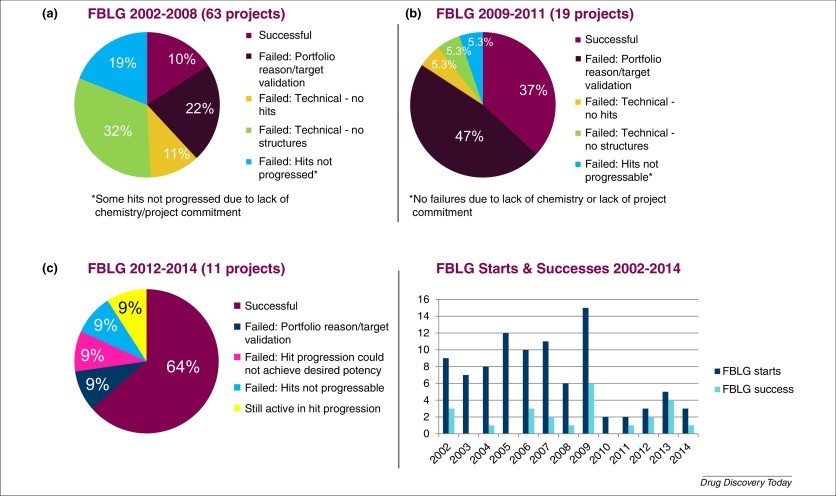
Figure 3: Fragment-based lead generation (FBLG) at AZ
(Source: Kettle et al., 2020)
These findings can be stated as stronger support with the fact that AZ requires the inclusion of 3D fragments in the construction of any fragment library to supplement more planar fragment hits in completely probing critical areas of interest. AZ FBLG teams require to transform the fragment hits into appropriate lead series for a wide variety of target classes, including targets with poorly projected draggability. Hence, it can be stated that the experiences and discoveries will be useful to anyone working in the area of fragment-based medication development.
Application of 5R strategy
From the existing literature it has found that the 5R framework, was developed and implemented where it was found that technical factors are prioritized for decision-making purposes through a few distinct stages (the stages are “the right target, suitable tissue, proper safety, right patient and right commercial potential”)( Blackburn, Fallah-Arani, and Porter, 2018).
Several evaluations on the subject of R&D productivity have been found. Research into disease biology and processes has been found as the new priority for AstraZeneca scientists. Target selection and validation, PK/PD model development, lead generation, patient classification, and biomarker development are areas in which the business has made significant efforts (Morgan et al., 2018).
The pharmaceutical sector, led by AstraZeneca, is investing more money into R&D, which translates to a reduction in funding for clinical trials. The marketing department is recommended to be considered for increasing successful sales growth. The R&D department must encounter other drug pipeline disorders, such as swine flu and cancer. Instead of chemicals, more agri-products must be used in research and development. As a result, patients get better care.
Increasing efficiency maintenance
AstraZeneca requires following a number of theories such as Total Production Management. Therefore, AstraZeneca requires following the Lean manufacturing processes effectively. TPM implementation should be a long-term strategy rather than a one-off fix. As a competitive advantage, preventative maintenance aims to eliminate any process interruptions by tracking and reporting failures and integrating the operators and maintenance personnel in the work involved (Nazy et al., 2021). In order to properly execute the idea, AZ must be diligent in following instructions and altering the corporate culture.
In order to be successful, a new way of thinking has to be adopted by all relevant departments. The concept of continuous development and the engagement of all employees must be highlighted. When it comes to management assistance, it’s no longer acceptable for a boss to reject an employee with improvement suggestions.
Technology Competence
The R&D department uses technology competency to develop new technologies at AZ. The following figure provides a clear view of the existing technology applications at AstraZeneca.
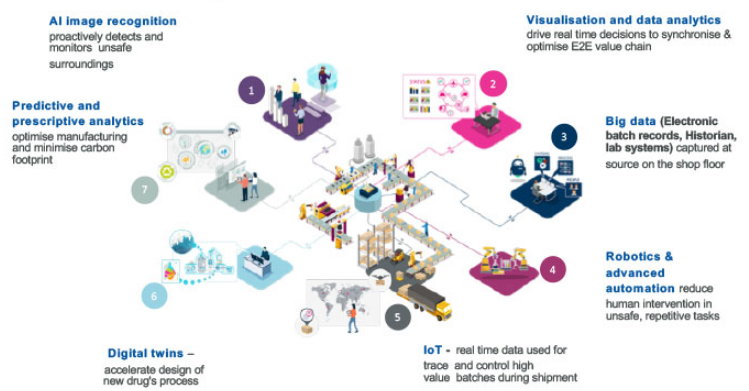
Figure 4: Innovation at AstraZeneca
(Source: ispe.org, 2019)
AstraZeneca must invest in mote higher-end technologies to strengthen each medication pipeline to dominate the pharmaceutical business. Product development for Armida and other cancer-fighting, gastro-intestinal-disease-alleviating, cardiovascular-disease-alleviating, asthma-and chronic obstructive pulmonary-disease-alleviating R&D products has improved dramatically (Temkin et al., 2021). Due to this, it is better equipped to take the lead in the industry in terms of technological proficiency.
To better understand, motivation theory is recommended to apply. The motivational theory is crucial, and it aims to describe the path from a desire to act to concrete action. Motivational management can have a significant impact on both individual and organizational performance if done appropriately.
Quality Assurance
Treatment quality should be assessed in different ways as well as should appropriately along with AstraZeneca’s main aim and objectives so that the company’s vision can be achieved appropriately. The quality assurance procedures should be used to improve clinical outcomes, minimize care variability, and bridge the gap between evidence-based recommendations and standard treatment (Hawthorne et al., 2019). A large number of quality indicators are developed from clinical practice guidelines.
Research findings and clinical consensus are included in evidence-based recommendations, and they provide a solid basis for quality improvement. There is not enough research indicating that following guidelines improves patient outcomes in mental health treatment, but this should not deter quality improvement efforts based on policies (Blackburn, Fallah-Arani, and Porter, 2018). Measures that aim to increase adherence to guidelines alone are too simple. Only if there are incentives to enhance the quality of care consistently can measurement-based quality improvement be effective.
Reflection
While preparing the consultancy report, I have adopted two basic stages, identifying the areas of improvement at AstraZeneca and stating adequate stages the company needs to follow.
However, I have experienced Anxiety as I believed AstraZeneca to be a significant customer. Perhaps more knowledgeable advisors have given it a more favorable evaluation than we have given it. Following our discussion with the customer, my emotional state shifted since they exhibited high confidence in our results and suggestions.
The Anxiety has also driven me to believe that we are constantly judged as newbies rather than a bunch of people who can bring fresh perspectives and ideas. This is one of the reasons why I felt threatened while doing the market research. According to my prior understanding, market surveys need a lot of resources in terms of statistical abilities and cash. However, the preparation of the present report has provided me with the additional capabilities to understand the specific places of improvement and what steps can provide it with increased potential for growth.
However, in certain stages more complex research was requested and we needed to achieve a particular number of participants to be declared statistically significant. As soon as we chose to work out with data, my gradual acceptance stage emerged. I believed that every voice from a client was important, no matter where it came from.
Project planning was one of the key events in the overall research. Since no one wanted to risk being penalized for being overdue for their report’s completion, they all agreed to record a substantial quantity of information weekly. The final report was put together from the many bits of information that had been gathered over the course of the preceding weeks.
However, the present reflection necessitates a separation from the events in question. The combination of the two elements allowed us to have the facts as they were and time to detach ourselves from events so that we could objectively reflect on results and expectations.
I had to put in the audience’s shoes and think about why AstraZeneca approached and why it was essential to them. I utilized a common finding in which it is stated that the firm was looking to improve its product and customer service standards based on client feedback. The customer frequently inquires about the matter on their own. I recognized that I needed to identify the target audience during our street research on the brand’s appeal. Furthermore, I might end up with skewed results by targeting a group that is not part of the brand’s target audience.
I think my stronger capabilities were to utilize the creation of the strategic plan following the exact opportunities for AstraZeneca.
Conclusion
In order to conclude it is to state that, AstraZeneca acquire major opportunities for improvement but its flawed reputation and the lack of in stronger structure are the key areas that are clogging its growth. As a strategic analysis report, the present report has greatly simplified the overall discussion and simply recommended a few stages that AZ needs to follow for further improvement. Additionally, it is stated that, along with newer approaches that AstraZeneca needs to follow, the report also emphasized a few potential approaches of AZ that the company needs to improve for further growth.
References
Blackburn, T., Fallah-Arani, F. and Porter, R.A., 2018. Current Trends in Drug Discovery–Young Scientists and Tomorrow’s Medicines: Highlights from a joint Society for Medicines Research and British Pharmacological Society meeting. Drugs of the Future, 43(8), pp.627-633.
Freedman, B.I., Burke, W., Divers, J., Eberhard, L., Gadegbeku, C.A., Gbadegesin, R., Hall, M.E., Jones-Smith, T., Knight, R., Kopp, J.B. and Kovesdy, C.P., 2021. Diagnosis, Education, and Care of Patients with APOL1-Associated Nephropathy: A Delphi Consensus and Systematic Review. Journal of the American Society of Nephrology, 32(7), pp.1765-1778.
Gutierrez, L., Cauchon, N.S., Christian, T.R., Giffin, M.J. and Abernathy, M.J., 2020. The confluence of innovation in therapeutics and regulation: recent CMC considerations. Journal of Pharmaceutical Sciences, 109(12), pp.3524-3534.
Hawthorne, G., Henderson, N., Hölttä, M., Stovold, C., Wåhlander, Å. and Wilson, A., 2019. Bioanalysis–but not as we knew it: an AstraZeneca perspective of the last 10 years evolution to meet a diversifying portfolio. Bioanalysis, 11(07), pp.595-599.
Holdgate, G., Embrey, K., Milbradt, A. and Davies, G., 2019. Biophysical methods in early drug discovery. ADMET and DMPK, 7(4), pp.222-241.
ispe.org, 2019. Digitization, Technologies, and More at 2019 ISPE Europe Annual Conference. [online] ISPE | International Society for Pharmaceutical Engineering. Available at: <https://ispe.org/pharmaceutical-engineering/july-august-2019/digitization-technologies-and-more-2019-ispe-europe> [Accessed 14 February 2022].
Kettle, J.G., Bagal, S.K., Bickerton, S., Bodnarchuk, M.S., Breed, J., Carbajo, R.J., Cassar, D.J., Chakraborty, A., Cosulich, S., Cumming, I. and Davies, M., 2020. Structure-based design and pharmacokinetic optimization of covalent allosteric inhibitors of the mutant GTPase KRASG12C. Journal of Medicinal Chemistry, 63(9), pp.4468-4483.
Montastruc, J.L., Lafaurie, M., de Canecaude, C., Montastruc, F., Bagheri, H., Durrieu, G. and Sommet, A., 2021. COVID-19 vaccines: a perspective from social pharmacology. Therapies, 76(4), pp.311-315.
Morgan, P., Brown, D.G., Lennard, S., Anderton, M.J., Barrett, J.C., Eriksson, U., Fidock, M., Hamren, B., Johnson, A., March, R.E. and Matcham, J., 2018. Impact of a five-dimensional framework on R&D productivity at AstraZeneca. Nature reviews Drug discovery, 17(3), pp.167-181.
Nazy, I.S.H.A.C., Sachs, U.J., Arnold, D.M., McKenzie, S.E., Choi, P., Althaus, K., Ahlen, M.T., Sharma, R., Grace, R.F. and Bakchoul, T., 2021. Recommendations for the clinical and laboratory diagnosis of vaccine-induced immune thrombotic thrombocytopenia (VITT) for SARS-CoV-2 infections: communication from the ISTH SSC Subcommittee on Platelet Immunology. J Thromb Haemost, 19(6), pp.1585-1588.
Runiewicz-Wardyn, M. and Eliashvili, T., 2022. Open Innovation Practices and Open Innovation Culture in the Life-Sciences Clusters. The Case of AstraZeneca. European Journal of Business and Management Research, 7(1), pp.35-43.
Temkin, S.M., Smeltzer, M.P., Dawkins, M.D., Boehmer, L.M., Senter, L., Black, D.R., Blank, S.V., Yemelyanova, A., Magliocco, A.M., Finkel, M.A. and Moore, T.E., 2021. Improving the quality of care for patients with advanced epithelial ovarian cancer: Program components, implementation barriers, and recommendations. Cancer.
www.astrazeneca.com, 2022. AstraZeneca – Research-Based BioPharmaceutical Company. [online] Astrazeneca.com. Available at: <https://www.astrazeneca.com/> [Accessed 14 February 2022].
www.astrazeneca.com, 2022. Transforming AstraZeneca’s R&D productivity. [online] Astrazeneca.com. Available at: <https://www.astrazeneca.com/what-science-can-do/topics/disease-understanding/transforming-astrazenecas-rd-productivity.html> [Accessed 14 February 2022].
 write
write