Introduction
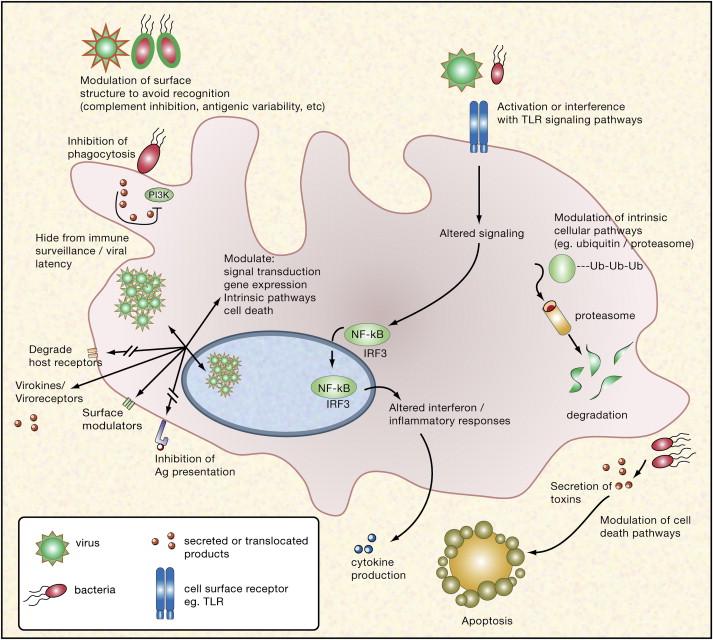
In the continuous battle between pathogens and the human immune system, pathogens have developed elaborate strategies to escape innate and specific defenses. It is essential to understand these immune evasion strategies to develop effective countermeasures. This essay discusses bacterial and viral pathogens’ strategies to evade the immune system. Thus, these mechanisms comprise a complex network that impedes our attempts to fight infectious diseases. The study of these evasion mechanisms not only improves our understanding of host-pathogen interactions but also opens therapeutic possibilities. An overview of the mechanisms is shown in the figure below:
Figure 1: An overview of the Various Mechanisms Used by Bacterial and Viral Pathogens to Overcome Innate and Acquired Immune Systems
Source: (Finlay & McFadden, 2006).
Antigenic Variation
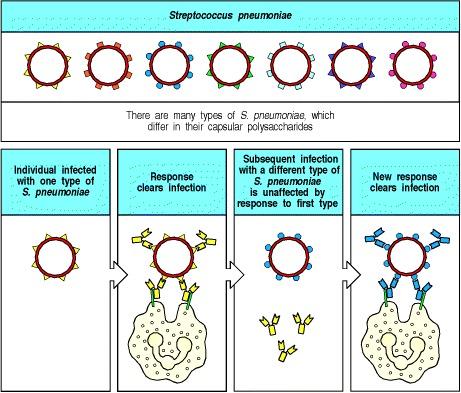
Antigenic variation is an important immune evasion strategy pathogens use to evade immune surveillance efficiently. This is especially important for extracellular pathogens when antibody production against their surface structures is the primary defense. Pathogens accomplish this evasion by modifying antigens or generating various antigenic types, as shown below (figure 2). For instance, Streptococcus pneumoniae has 84 known serotypes based on capsular polysaccharides. One serotype infection confers type-specific immunity, protecting an individual from reinfection by the same serotype but not other types (Finlay & McFadden, 2006). This peculiar adaptation makes it possible for the same pathogen to cause repeated diseases in an individual.
Figure 2: Host defense against Streptococcus pneumoniae is type-specific
Source: (Janeway et al., 2001).
Host Molecules Mimicry (Cell Wall Modification and Capsule Production)
Pathogenic bacteria use clever tricks to hide from the alert human immune system. The cell wall, the primary target of immune defenses, is modified by a widely used mysterious technique. As shown in the figure below (figure 3), gram-negative organisms such as E. coli and S. enterica acylate lipid A change the cell wall’s charge to repel host-produced antimicrobial peptides (AMPs). Some Gram-positive pathogens, such as Staphylococcus aureus, decrease teichoic acid negativity by D-alanylation. A commensal in the upper respiratory tract turned pathogen, Neisseria meningitidis takes a physical “cloak”—a polysaccharide capsule (Matsuura, 2013). This capsule protects the bacterium from immune defenses, and its production is strictly controlled during infection phases. Importantly, some bacterial capsules resemble mammalian cell surface polysaccharides and may be responsible for severe diseases, such as neural complications.
Figure 3: Orientation of LipidA within the gram-negative cell wall.
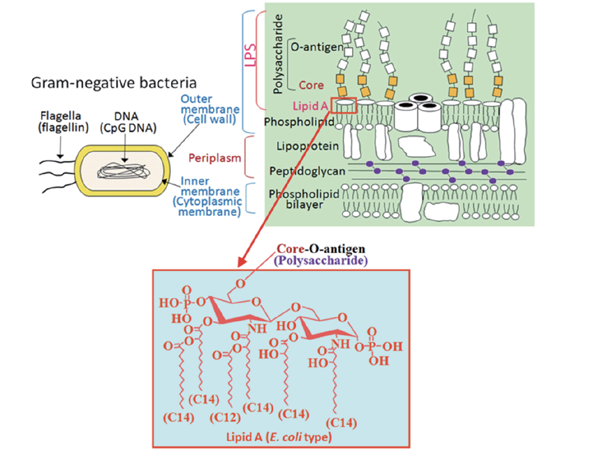
Source: (Zhang et al., 2015)
Inhibition of Phagocytosis
Bacterial pathogens strategically avoid phagocytosis, an important immune process, as shown above (figure 1). Interestingly, Yersinia species, including Y. pestis, which causes plague, use the complex T3SS (Mota & Cornelis, 2005). T3SS delivers effectors, such as YopH, a tyrosine phosphatase that disrupts actin dynamics vital to phagocytosis. Furthermore, YopE, a Rho GTPase-activating protein, YopO, a serine/threonine kinase, and YopT, a cysteine protease, coordinate and manipulate the host actin regulators. By diminishing phagocytic activity in this way, these bacteria can evade internalization and killing
Escape from Complement System
The complement system is essential to innate immunity, coordinating antimicrobial activities through separate cascades. Aware of its importance, viruses use clever ways to block this mechanism—viruses such as human cytomegalovirus cause cellular complement inhibitors such as DAF and MCP expression in infected cells. Moreover, viruses like HIV, HTLV-I, and vaccinia have host inhibitors integrated into their envelopes (Blom, 2004). Therefore, this host complement inhibitor hijacking can be used as an anti-complement virus defense mechanism.
Suppression of Cytokine Production
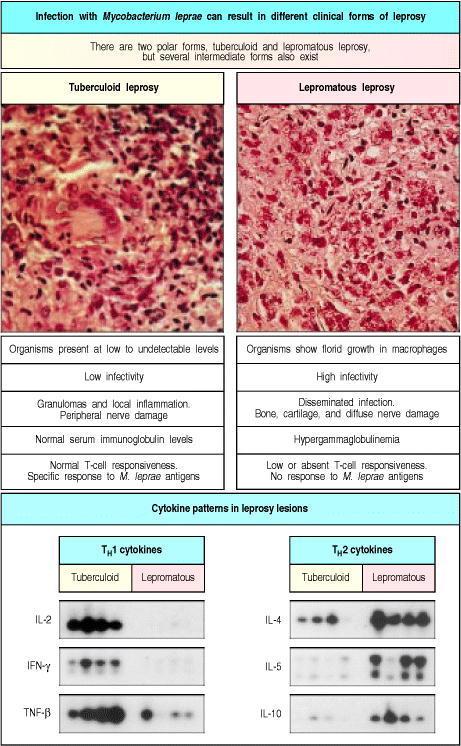
Pathogens use multiple strategies to inhibit cytokine generation, resulting in persistent diseases. For instance, staphylococci release superantigens like toxic shock syndrome toxin-1, causing overall T cell activation. These activated T cells, after quickly multiplying, commit suicide through apoptosis, leading to total immunosuppression. Complex immune modulation characterizes leprosy caused by Mycobacterium leprae. There is profound cell-mediated immune suppression in lepromatous leprosy, resulting in anergy. On the other hand, tuberculoid leprosy features strong cell-mediated immunity, controlling the infection. One possible mechanism for the distinctive disease phenotypes is the TH1 to TH2 cell ratio, cytokine-driven by CD8 T cells (Figure 4).
Figure 4: T-cell and macrophage responses to Mycobacterium leprae are sharply different in the two polar forms of leprosy
Source: (Janeway et al., 2001).
Manipulation of Host Signaling Pathways
Current research is focused on the intricate regulation of viruses in host signaling pathways. As shown above (Figure 1), they intentionally target recognition or downstream Toll-like receptor (TLR) signaling pathways important in cells to detect viral infections. For instance, poxviruses have proteins that block TLR pathways, stopping immune responses (Harte et al., 2003). However, understanding the different roles of TLR family members during viral pathogenesis is still complicated by overlapping redundancies. Clarifying these signaling pathways helps better understand host-pathogen interactions and possible therapeutic targets.
Inhibition of Apoptosis by Viral Proteins
By strategically interfering with the host’s apoptotic machinery, viruses can circumvent programmed cell death and thus ensure their survival. Apoptosis, one of the main mechanisms responsible for eliminating virus-infected cells, is mediated by cytotoxic T cells and natural killer cells delivering lethal granzyme granules or triggering TNF and Fas ligands, as shown below (Figure 5). Virus proteins can increase the activity of Bcl, an anti-apoptotic protein, or suppress its natural degradation. Viral proteins may antagonize the pro-apoptotic antioncogene P53 (Hilleman, 2004). In particular, oncogenic viruses such as HPV, EBV, KSHV, and HTLV-1 are known to activate the PI3K-Akt signaling pathway, preventing apoptosis and autophagy while providing a favorable environment for their replication (Zhang et al., 2015). These viral evasion strategies usurp the host’s defense mechanisms to cause persistent infection and replication.
Figure 5: Blockade of apoptosis by altering cellular events.
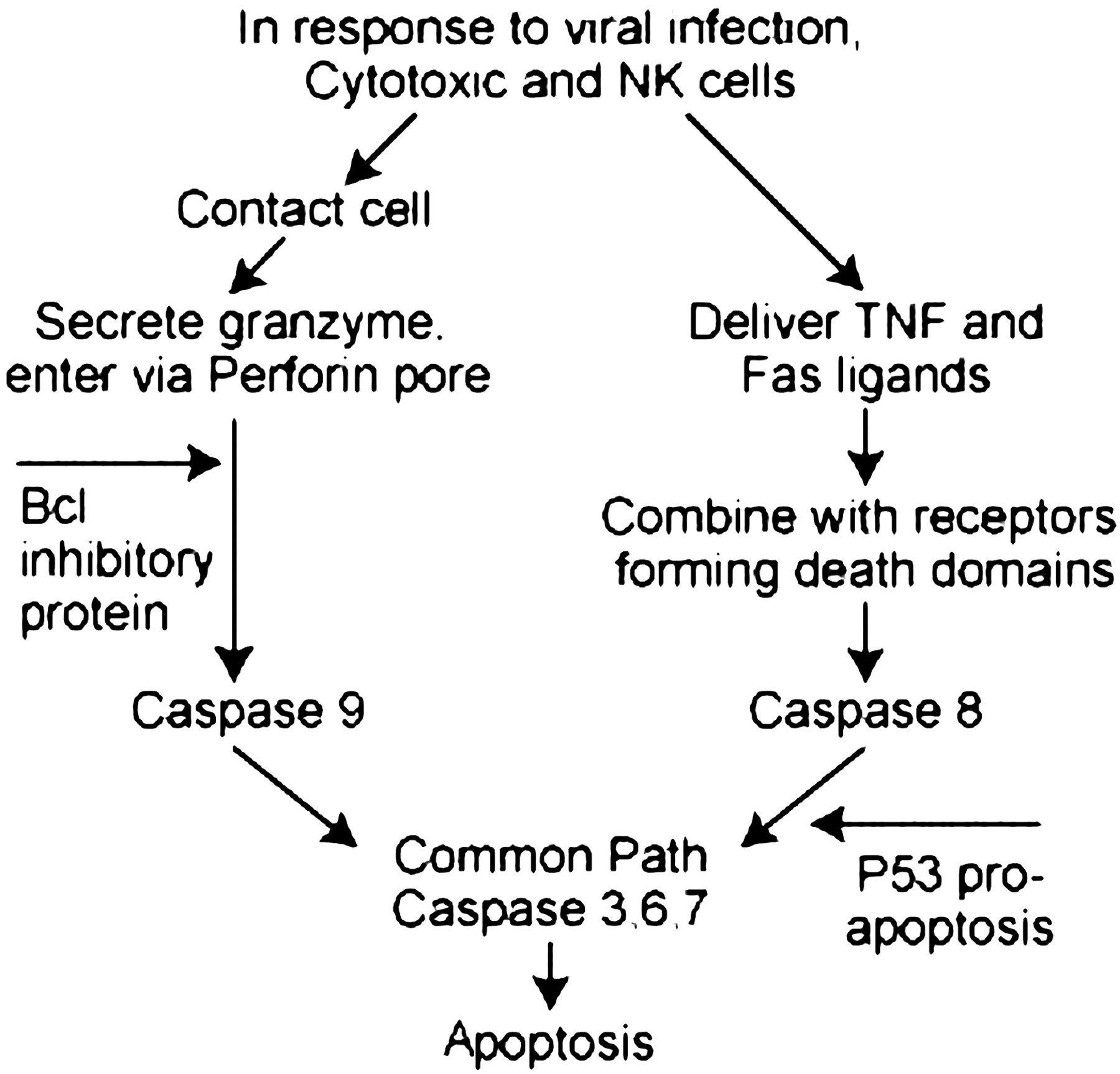
Source: (Hilleman, 2004).
Formation of Biofilms
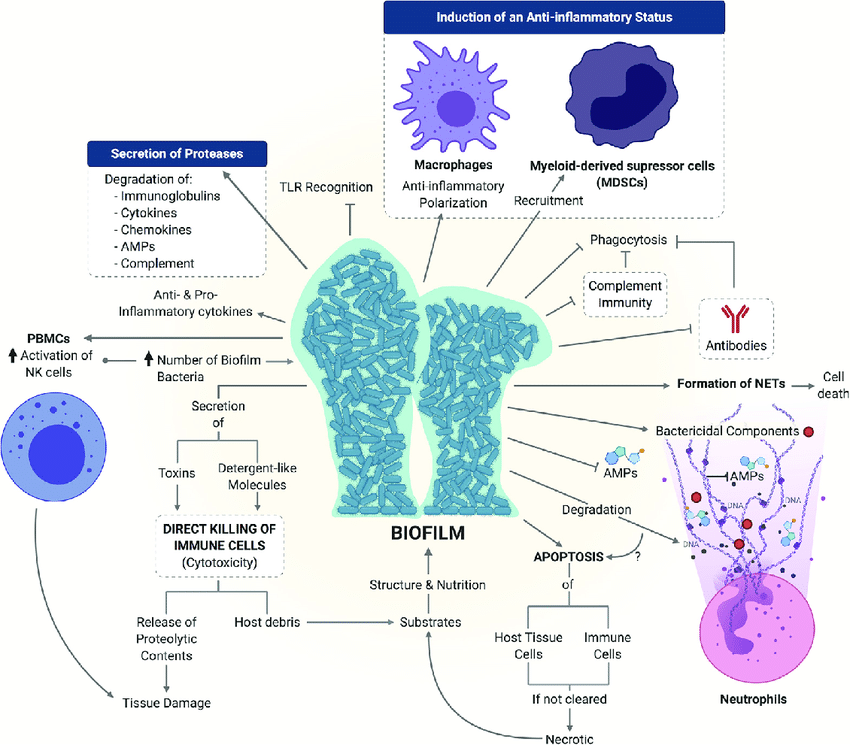
Bacterial biofilms utilize complex mechanisms to circumvent host immune responses that lead to chronic infections. Interference of humoral immunity is a hallmark in that staphylococcal biofilms, not impeding the diffusion of humoral components, profit from their vast biomass to dilute target antibodies, blocking opsonophagocytosis (Cerca et al., 2006). In addition, biofilms coordinate toxin release, disrupting identification and maneuvering the inflammatory state of summon MDSCs, MΦs, and neutrophils, as shown below (figure 6). The overexpression of alkaline proteases and elastases by Pseudomonas aeruginosa biofilms directly deactivates the complement proteins, which illustrates the broad spectrum of evasion mechanisms occurring in persistent biofilm infections (Mulcahy et al., 2014). Treatment is only possible after physically separating and removing infected tissue or medical equipment.
Figure 6: Biofilm mechanisms of immune evasion
Source: (Guzman-Soto et al., 2021).
Conclusion
The immune evasion strategies of infectious agents form a complex and diverse spectrum of tactics that significantly threaten human wellness. From bacterial camouflage through cell wall modification to viral interference with apoptosis, pathogens will never stop evolving defenses to escape immune system surveillance. Deciphering such complex avoidance mechanisms is crucial for developing proper preventive and therapeutic treatment strategies against infectious diseases. With the ongoing evolution of research, the subtle tango between pathogens and the immune system persists, underscoring the importance of constant vigilance and ingenuity in our tactics to stay one step ahead of these clever opponents.
References
Blom, A. M. (2004). Strategies developed by bacteria and virus for protection from the human complement system. Scandinavian Journal of Clinical and Laboratory Investigation, 64(5), 479-496.
Celli, J., & Finlay, B. B. (2002). Bacterial avoidance of phagocytosis. Trends in microbiology, 10(5), 232-237.
Cerca, N., Jefferson, K. K., Oliveira, R., Pier, G. B., & Azeredo, J. (2006). Comparative antibody-mediated phagocytosis of Staphylococcus epidermidis cells grown in a biofilm or in the planktonic state. Infection and immunity, 74(8), 4849-4855.
Finlay, B. B., & McFadden, G. (2006). Anti-immunology: evasion of the host immune system by bacterial and viral pathogens. Cell, 124(4), 767-782.
Guzman-Soto, I., McTiernan, C., Gonzalez-Gomez, M., Ross, A., Gupta, K., Suuronen, E. J., … & Alarcon, E. I. (2021). Mimicking biofilm formation and development: Recent progress in in vitro and in vivo biofilm models. Iscience, 24(5).
Harte, M. T., Haga, I. R., Maloney, G., Gray, P., Reading, P. C., Bartlett, N. W., … & O’Neill, L. A. (2003). The poxvirus protein A52R targets Toll-like receptor signaling complexes to suppress host defense. The Journal of experimental medicine, 197(3), 343-351.
Hilleman, M. R. (2004). Colloquium Paper: Therapeutic Vaccines: Realities of Today and Hopes for Tomorrow: Strategies and mechanisms for host and pathogen survival in acute and persistent viral infections. Proceedings of the National Academy of Sciences of the United States of America, 101(Suppl 2), 14560.
Janeway CA Jr, Travers P, Walport M, et al. (2001). Immunobiology: The Immune System in Health and Disease. 5th edition. New York: Garland Science; 2001. Pathogens have evolved various means of evading or subverting normal host defenses. Available from: https://www.ncbi.nlm.nih.gov/books/NBK27176/
Matsuura, M. (2013). Structural modifications of bacterial lipopolysaccharide that facilitate gram-negative bacteria evasion of host innate immunity. Frontiers in immunology, 4, 109.
Mota, L. J., & Cornelis, G. R. (2005). The bacterial injection kit: type III secretion systems. Annals of medicine, 37(4), 234-249.
Mulcahy, L. R., Isabella, V. M., & Lewis, K. (2014). Pseudomonas aeruginosa biofilms in disease. Microbial ecology, 68, 1-12.
Zhang, L., Wu, J., Ling, M. T., Zhao, L., & Zhao, K. N. (2015). The role of the PI3K/Akt/mTOR signalling pathway in human cancers induced by infection with human papillomaviruses. Molecular cancer, 14(1), 1-13.
 write
write