Abstract
Cadmium (Cd) is an unnecessary poisonous metal for most life forms. It poses several environmental dangers, some of which can lead to plant problems and human illness. Due to its tenacious nature, it can accumulate in several human bodily organs. Mining, smelting, and refining are just some of the human-caused activities contributing to Cd’s introduction into the environment. Plastics, pigments, enamels, ceramics, and steel plating are only a few industrial uses. Cd toxicity can be caused by exposure to Cd in three common forms: water, air, and soil. Cd is a byproduct of some manufacturing operations. The kidney, lungs, bones, and liver are just a few of the organs affected due to exposure to cadmium. To eliminate these public health concerns, it is essential to ensure that water systems are well treated to eliminate cadmium contamination of drinking water. Treatment systems that can remove cadmium from wastewater samples are chemical procedures, membrane methods, ion exchange methods, and adsorption techniques. Some several analytical techniques/methods can be used for the analysis of cadmium from wastewater samples; Atomic absorption spectrophotometry (AAS), Inductively coupled plasma mass spectrometry (ICP-MS), Atomic fluorescence spectroscopy (AFS) and X-ray fluorescence spectrometry (XRF).
Introduction
Cadmium (Cd) is a soft, malleable, silver-white metal that is part of group IIb in the periodic table, along with zinc and mercury. It has a high vapor pressure despite its low melting point (320.9 °C) and boiling point (765 °C). Cadmium quickly oxidizes into cadmium oxide when exposed to air. Cd is a very toxic environmental pollutant that has devastating impacts on ecosystems and human health. To protect the environment, it is crucial to limit cadmium production and discharge to near-zero levels. Over the years, scientists have investigated a number of approaches that can be used to keep tabs on and regulate candium levels. The purpose of this paper is to provide a comprehensive discussion of cadmium, including its origins, impacts on human health and the environment, potential removal methods, and analysis procedures in wastewater. The sample for the analysis of cadmium in this report will be river water from a location close to a mining operation in China.
Sources
As its purity is not present in nature, cadmium is a relatively uncommon element (0.2 mg/kg in the earth’s crust). Usually, it’s found near sulfide ores of metals like zinc, lead, and copper. Commercial production of cadmium did not begin until the 20th century. As a byproduct of zinc manufacturing, its availability is heavily dependent on the metal’s overall output. Before World War One, cadmium was rarely extracted from zinc and other nonferrous metals facilities, leading to decades of unchecked environmental poisoning. From only 20 tonnes in the 1920s, annual global production of cadmium climbed to around 12,000 tonnes between 1960 and 1969, 17,000 tonnes between 1970 and 1984, and around 20,000 tonnes on average per year since 1987 (Genchi et al., 2020).
Cadmium’s usage has shifted in recent decades. Historically, cadmium’s primary use has been in metal electroplating and the production of pigments and plastic stabilizers. Almost 50% of global cadmium consumption in 1960 came from the engineering coatings and plating sector; by 1990, this had dropped to 8%. (Hayat et al., 2019). Currently, nickel-cadmium battery production accounts for 55% of cadmium use; with the rise of rechargeable batteries and the promise of electric vehicles, this percentage is only projected to rise (Sall et al., 2020). In nickel-cadmium batteries, for instance, cadmium consumption increased from 3,000 tonnes in 1980 to 9,000 tonnes in 1990. (Kubier et al., 2019). This explosive expansion has more than made up for a 20% decline in pigment use, an 8% drop in plating, and a 10% drop in the use of stabilizers. Cadmium has found a wide variety of uses in the electronics, communications, power generation, and aerospace industries, among many others.
Impacts on the environment and human health.
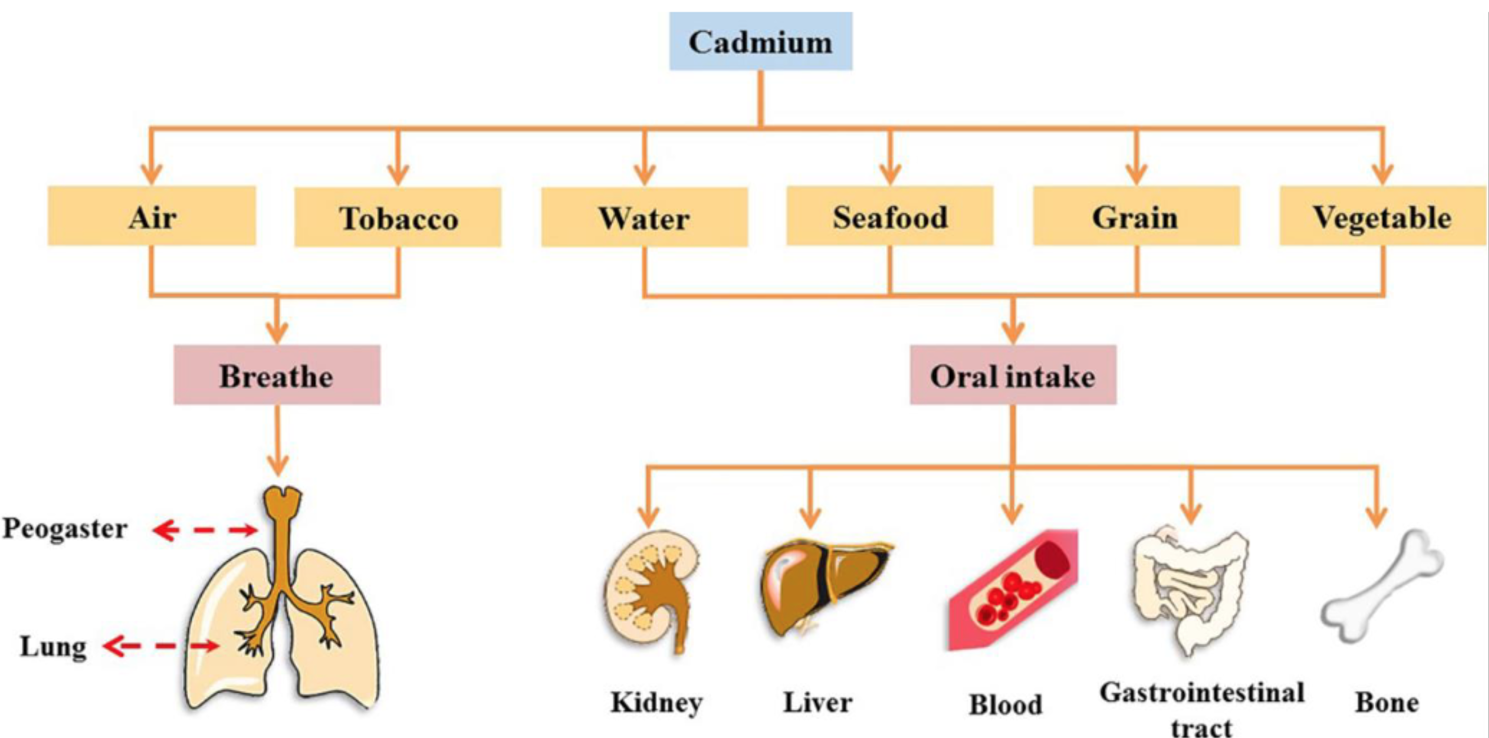
Cadmium is a contaminant that has been linked to negative health effects in humans. Its mining and smelting, along with other human-made pathways like the use of phosphate fertilizers and the presence of NiCd batteries, plating, pigments, and plastics, have all contributed to its widespread atmospheric dispersal (Hayat et al., 2019). Air, tainted food, and water all contribute to people’s Cd exposure. Cd exposure, even at low levels, has been linked to health problems such as kidney damage, liver damage, skeletal system damage, cardiovascular system damage, and loss of vision and hearing (Kubier et al., 2029). Cadmium has also been related to adverse effects during pregnancy or the outcome of a pregnancy. (Genchi et al., 2020). This happens because changes in embryonic gene expression lead to aberrant mutagenesis of genetic makeup in both the mother and the infant (Sall et al., 2020).
Metal fume fever-like symptoms have been reported in people exposed to modest amounts of newly generated cadmium fume for less than an hour. One of the good things about this effect is that complete recovery often happens within a few days. In 10-15% of instances, after a latency period of several hours, mortality occurs due to chemical pneumonitis caused by prolonged or very high levels of exposure (Sall et al., 2020). Long-term cadmium exposure in the workplace has been linked to respiratory problems such as bronchitis, obstructive lung disease, and emphysema among workers.
Treatment systems
Cd-containing effluents from mines and factories can be treated using a number of different physical and chemical processes. Chemical procedures, membrane methods, ion exchange methods, and adsorption techniques are some examples of these approaches.
1. Chemical methods
a) Precipitation
Around 75% of electroplating facilities use precipitation of metals as insoluble hydroxides, carbonates, or sulfides to remove metals from effluent. Due to its cheap cost and simple execution, heavy metal hydroxide precipitation has become the standard treatment procedure (Pyrzynska, 2019). Increasing the effluent pH with lime (CaO) or caustic soda (NaOH) causes the heavy metals to precipitate as their respective hydroxides, rendering them immobile. The addition of barium acetate to plating effluents is one method for removing cadmium, as described by Hou et al. (2022). Hasan (2021) identified the diisobutyldithiophosphinate compound of cadmium as a selective precipitant for its removal.
b) Cementration
According to Pyrzynska. (2019), cementation with zinc powder can be used to remove cadmium from solutions. The optimal pH was determined to be between 4.5 and 5.5. The pace of the reaction was nearly first order with regard to the concentration of cadmium ions and the amount of zinc present. Sodium dodecyl sulfonate, an anionic surfactant, was the only one of those tested that increased the cementation rate of cadmium by zinc powder significantly. Because Cd-EDTA chelates have a larger redox potential than free cadmium ions, their synthesis in aqueous solutions of ethylene-diaminetetraacetic acid (EDTA) impeded cadmium removal by zinc.
2. Ion exchange technique
Chemical reactions take place between a dissolved electrolyte and an insoluble electrolyte during an ion exchange operation. Lewatit TP260, a cationic (di-Na+) ion exchange resin, was studied for its ability to adsorb cadmium(II) from an aqueous sulphate media. The absorption of cadmium(II) was reported to be controlled by particle diffusion, while adsorption onto the resin followed the Langmuir equation (Hasan et al., 2021). The initial metal adsorption on the resin might be explained by the moving border particle diffusion concept. Adsorption studies on cadmium in HBr and NaBr-H2SO4 medium using N5O3 (N, N-di(sec-octyl) acetamide) levextrel resin have been conducted. Adsorption of cadmium by N5O3 levextrel resin was found to occur in a pseudo-first-order fashion and conform to the Freundlich isothermal adsorption equation (Pyrzynska, 2019). Due to the need for chemicals in the resin regeneration process, ion exchange systems often have a high operational cost in addition to a high initial cost. As a result, it is not commonly used to remove Cd from water supplies.
3. Membrane separation technique
In the liquid membrane method, an organic membrane and an aqueous internal phase are dispersed in a continuous external phase. When a carrier is added to the membrane phase, the solute in the external phase is made soluble in what would otherwise be an inert medium. At the interface between the membrane and interior phases, a stripping agent reacts with the transferred solute, trapping it in the latter (Hou et al., 2022). Demulsifiers are used to separate the emulsion phases so that the oil can be reused in the emulsification process. Membrane methods including liquid membrane, hollow fiber supported liquid membrane, supported liquid membrane, and emulsion liquid membrane are used to purge cadmium from water.
4. Adsorption technique
Adsorption is a successful approach for cleaning up heavy and harmful ions. Throughout the past few decades, there has been a surge in efforts to create new adsorbents and improve the efficiency of current ones. Due to its large specific surface area, high adsorption capacity, and particular surface chemical characteristics, activated carbon is widely employed in wastewater treatment for the removal of organic and inorganic contaminants (Pyrzynska, 2019). Many sub standard materials and hazardous wastewater have been evaluated for their cation/anion absorption behavior in an effort to produce cost-effective adsorbents.
Adsorption is one of the most often used technologies because of its low entry barrier, high efficiency, straightforward recovery, and low cost. For this application, an ideal adsorbent would have a large surface area, a large adsorption capacity, strong mechanical stability, and be easily regenerable (Pyrzynska, 2019). The efficiency of the adsorption process is also strongly affected by process parameters like the pH of the solution, the temperature of the adsorbent, the contact time, and the adsorbent dosage.
Monitoring strategy
When it comes to protecting public health and stopping pollution, knowing how much cadmium is in wastewater is crucial. Changes in cadmium levels over time can be detected, and probable sources of contamination can be identified with the help of a well-designed monitoring approach. Sampling is the first of several phases in a comprehensive plan for cadmium monitoring in wastewater. Choosing how often and where to take samples is the first and most crucial step. The danger of the wastewater source and any applicable legislation or guidelines will determine how often samples should be taken. Any potential sources of contamination, including industrial discharges or urban runoff, should be considered when selecting sample locations. A Chinese mining firm serves as our hypothetical case study’s sampling location. Cadmium contamination of the river near the company’s headquarters is a major concern.
Step two is gathering samples for analysis. This process requires taking samples of wastewater in accordance with predetermined guidelines. The methodology for collecting samples should outline how and where the samples will be stored. It’s important to collect samples in sufficient numbers to allow for repetitions and analysis, and at times and locations that are typical of the whole. Keeping the samples in good condition is essential to avoiding cadmium loss during shipping and storage. The samples may need to be refrigerated or a preservative added, like nitric acid.
Analyzing the data is the third phase. Using an established technique, like inductively coupled plasma mass spectrometry (ICP-MS) or atomic absorption spectrophotometry, to analyze the samples for cadmium level is recommended . An accredited lab with strict quality control procedures should conduct the analysis. This is followed by the installation of quality control mechanisms to guarantee the analysis’ precision and correctness. One method is to use certified reference materials; another is to analyze duplicate samples; a third is to spike samples with known quantities of cadmium.
The data is interpreted after it has been analyzed. Trends and patterns in cadmium concentrations throughout time are uncovered by analyzing the collected data. We then look into any discrepancies between the results and the applicable regulations or recommendations. The cadmium DL in our hypothetical situation is 0.005 g/L. Out of a total of 24 observations, 16 were below the detection limit (BDL) before the mining operation was established. After the data mining operation, however, there is a dramatic shift in the numbers, suggesting that 40 of the original 64 observations are now below the detection limit.
Lastly, the results of the monitoring program must be documented and reported to the public, regulatory authorities, and wastewater treatment operators by the environment manager. The report should be easy to read and understand, as well as suggest next steps if needed. An environmental manager can get trustworthy information while analyzing wastewater samples containing cadmium if they use this method of monitoring.
Analytical techniques/methods used for the analysis of water samples containing cadmium
There are several analytical techniques/methods that can be used for the analysis of water samples containing cadmium. These methods include;
1. Atomic absorption spectrophotometry (AAS):
The analysis of water samples for cadmium is commonly done using AAS. To determine how much cadmium is in the water, this technique makes use of the time-tested scientific principle of light absorption by the sample (Cadorim et al., 2019). With AAS, cadmium concentrations as low as a few parts per billion can be detected with great precision (ppb). There are a number of stages to this procedure. Preparing a sample is the first stage. Filtration or acidification of the water sample is performed prior to analysis, depending on the sample type and technique (Halko et al., 2022). The sample is next diluted with an appropriate solvent so that the concentration of cadmium is within the linear range of the AAS.
The prepared sample is then atomized by being placed in a graphite or metal furnace at high temperature. For thorough atomization, the furnace is kept at a specific temperature. The third step, absorption, involves irradiating the sample with light from a hollow cathode lamp that generates a narrow spectrum of radiation at the wavelength peculiar to cadmium. Radiation is absorbed by the cadmium atoms present in the sample, and the rate at which it is absorbed is proportional to the cadmium concentration (Cadorim et al., 2019). The detection phase follows the absorption phase. At this point, the radiation intensity after passing through the sample is measured using a photodetector. The amount of radiation absorbed by the cadmium atoms in the sample is proportional to the strength of the signal produced by the detector.
In order to accurately determine the quantity of cadmium in a sample, a series of standard solutions with known concentrations are created and evaluated in the same way. Each standard solution’s signal strength is measured, and a calibration curve is generated by plotting the signal strength against the known cadmium content (Cadorim et al., 2019). Quantification is the final stage. By comparing the signal generated by the sample to the calibration curve, the cadmium concentration in the water sample may be calculated. The formula used to determine the concentration involves the dilution factor and the sample volume.
2. Inductively coupled plasma mass spectrometry (ICP-MS):
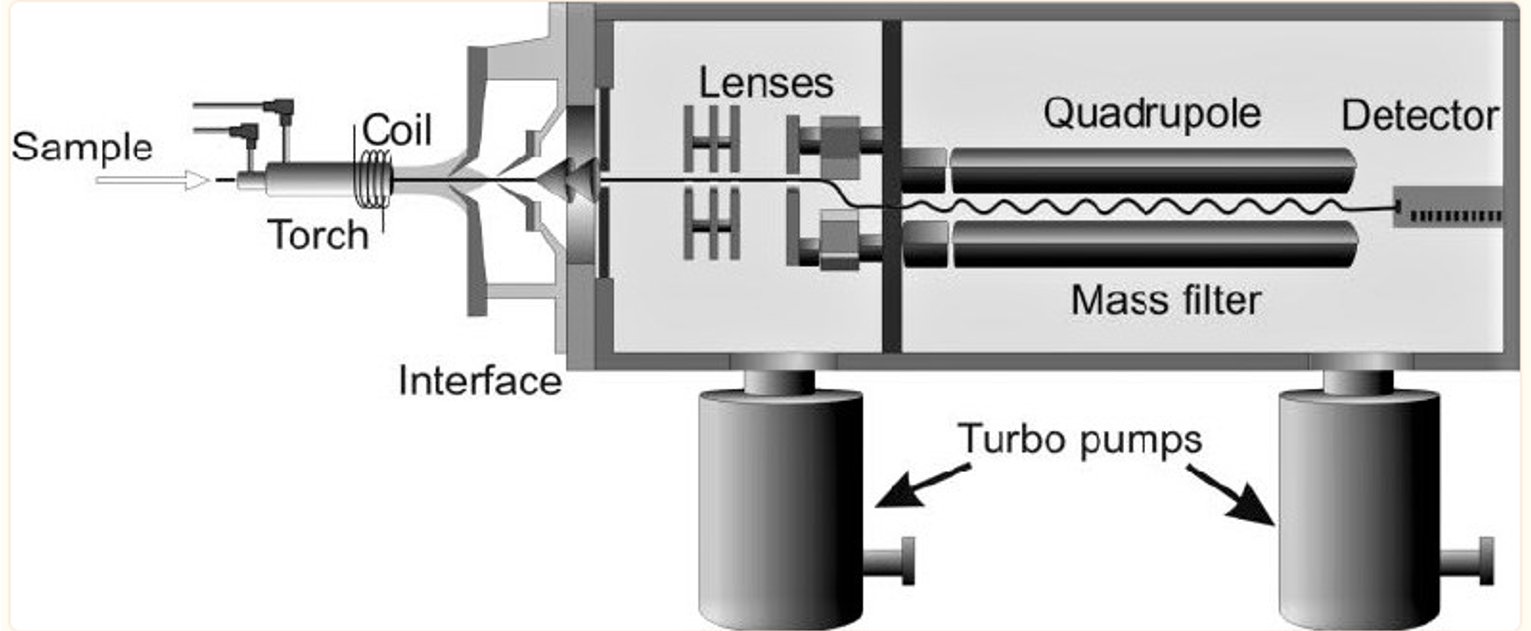
ICP-MS is an extremely sensitive and specific technique for analyzing water samples for cadmium. The sample is first ionized in an inductively coupled plasma, and then the cadmium ions are detected using mass spectrometry. ICP-MS is sensitive enough to identify cadmium at levels as small as a few ppt (ppt). Sample introduction system, inductively coupled plasma (ICP), interface, ion optics, mass analyzer, and detector are the six main parts that make up a single quadrupole ICP-MS (Wilschefski & Baxter, 2019). A basic diagram of the tool is shown in Figure 2. The sample introduction mechanism nebulizes the liquid sample to produce a fine aerosol, which is then introduced to the argon plasma (Lan et al., 2022). The material is atomized and ionized in a high-temperature plasma, and the resulting ions are collected in a set of electrostatic lenses known as the ion optics (Wilschefski & Baxter, 2019). The quadrupole mass analyzer receives the focused ion beam thanks to the ion optics. Ions are sorted by their mass-to-charge ratio (m/z) in the mass analyzer before being sent to the detector for analysis.
3. Atomic fluorescence spectroscopy (AFS)
The detection of cadmium in water samples by use of AFS is a highly accurate and selective technique. The amount of cadmium in the water is determined by measuring the fluorescence produced when the sample is excited by a light source (Zhou et al., 2019). There are two phases of atomic fluorescence. In the first phase, photons from an intense monochromatic light source are used to excite atoms in a sample. Excited electrons gain even more energy in Stage 2. Once the electrons return to their stable state, they release energy in the form of photons. A spectroscope is used to measure the electromagnetic energy emitted during this process, providing a means of determining the relative abundance of individual substances (Halko et al., 2022). AFS’s key benefit is its great specificity; it can notice certain elements even in very low quantities, which makes it perfect for detecting small amounts of contaminated metals (Zhou et al., 2019). As a result of its sensitivity, AFS may be used to test for a wide range of metals. It’s also a straightforward technique, so it’s possible to test a lot of samples in a short amount of time and get useful results.
4. X-ray fluorescence spectrometry (XRF):
X-ray fluorescence (XRF) is a non-destructive technique for analyzing water samples for cadmium. X-rays are used to excite the sample, which then emits its own X-rays with a specific signature (Margui et al., 2022). The amount of cadmium in the water directly correlates to the strength of the emitted X-rays. Total-reflec- tion analysis requires samples to be presented as thin films. The sample volume, from 5 to 50 milliliters, is deposited on a reflective carrier and dried. X-ray photons are almost totally absorbed into thin objects at very low glancing angles of the primary X-ray beam, which is exploited by the TXRF system (Margui et al., 2022). As a result, detection limits at the mgL level are enhanced due to the lack of a high background typically seen as a result of dispersion from the sample support.
The overall sensitivity, selectivity, accuracy, and precision needed for a given application, as well as the availability of tools and materials, will determine which analytical approach is chosen. Choosing a validated method that satisfies regulatory needs for cadmium assessment in water samples is crucial.
Conclusion
Cadmium is extremely harmful to humans and should be eliminated r maintained at the minimal levels in the environment. Cd is efficiently maintained in the body after absorption and builds up over time. Proximal tubular cells in the kidney are particularly vulnerable to Cd’s toxicity. Bone demineralization may come from Cd’s direct effects on bones or its indirect effects on the kidneys. Exposure to high levels of airborne Cd in the workplace has been linked to diminished lung function and an increased risk of lung cancer. All of these outcomes have been shown in populations exposed to moderately high levels of Cd in either industrial or substantially polluted settings. Therefore, it is essential to be vigilant in analysis, and monitoring of codmium concentration at all times.
References
Cadorim, H. R., Schneider, M., Hinz, J., Luvizon, F., Dias, A. N., Carasek, E., & Welz, B. (2019). Effective and high-throughput analytical methodology for the determination of lead and cadmium in water samples by disposable pipette extraction coupled with high-resolution continuum source graphite furnace atomic absorption spectrometry (HR-CS GF AAS). Analytical Letters, 52(13), 2133-2149. https://www.tandfonline.com/doi/abs/10.1080/00032719.2019.1596117
Genchi, G., Sinicropi, M. S., Lauria, G., Carocci, A., & Catalano, A. (2020). The effects of cadmium toxicity. International journal of environmental research and public health, 17(11), 3782. https://www.mdpi.com/726598
Halko, R., Tuček, J., Chovancová, K., & Andruch, V. (2022). Some green approaches in atomic absorption spectrometry. The last 10 years. Applied Spectroscopy Reviews, 1-48. https://www.tandfonline.com/doi/abs/10.1080/05704928.2022.2148685
Hayat, M. T., Nauman, M., Nazir, N., Ali, S., & Bangash, N. (2019). Environmental hazards of cadmium: past, present, and future. In Cadmium toxicity and tolerance in plants (pp. 163-183). Academic Press. https://www.sciencedirect.com/science/article/pii/B9780128148648000073
Hasan, M. N., Salman, M. S., Islam, A., Znad, H., & Hasan, M. M. (2021). Sustainable composite sensor material for optical cadmium (II) monitoring and capturing from wastewater. Microchemical Journal, 161, 105800. https://www.sciencedirect.com/science/article/pii/S0026265X20337401
Hou, C., Zhao, J., Zhang, Y., Qian, Y., Chen, J., Yang, M., … & Zhou, X. (2022). Enhanced simultaneous removal of cadmium, lead, and acetochlor in hyporheic zones with calcium peroxide coupled with zero-valent iron: Mechanisms and application. Chemical Engineering Journal, 427, 130900. https://www.sciencedirect.com/science/article/pii/S1385894721024840
Kubier, A., Wilkin, R. T., & Pichler, T. (2019). Cadmium in soils and groundwater: a review. Applied Geochemistry, 108, 104388. https://www.sciencedirect.com/science/article/pii/S0883292719301805
Lan, G., Li, X., Jia, H., Yu, X., Wang, Z., Yao, J., & Mao, X. (2022). Fast and Sensitive Determination of Cadmium and Selenium in Rice by Direct Sampling Electrothermal Vaporization Inductively Coupled Plasma Mass Spectrometry. Molecules, 27(23), 8176. https://www.mdpi.com/1420-3049/27/23/8176
Marguí, E., Queralt, I., & Almeida, E. (2022). X-ray fluorescence spectrometry for environmental analysis: Basic principles, instrumentation, applications and recent trends. Chemosphere, 135006. https://www.sciencedirect.com/science/article/pii/S0045653522014990
Pyrzynska, K. (2019). Removal of cadmium from wastewaters with low-cost adsorbents. Journal of Environmental Chemical Engineering, 7(1), 102795. https://www.sciencedirect.com/science/article/pii/S2213343718307206
Sall, M. L., Diaw, A. K. D., Gningue-Sall, D., Efremova Aaron, S., & Aaron, J. J. (2020). Toxic heavy metals: impact on the environment and human health, and treatment with conducting organic polymers, a review. Environmental Science and Pollution Research, 27, 29927-29942. https://link.springer.com/article/10.1007/s11356-020-09354-3
Wang, Z., Sun, Y., Yao, W., Ba, Q., & Wang, H. (2021). Effects of cadmium exposure on the immune system and immunoregulation. Frontiers in Immunology, 12, 695484. https://www.frontiersin.org/articles/10.3389/fimmu.2021.695484/full
Wilschefski, S. C., & Baxter, M. R. (2019). Inductively coupled plasma mass spectrometry: introduction to analytical aspects. The Clinical Biochemist Reviews, 40(3), 115. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6719745/
Zhou, J., Deng, D., Su, Y., & Lv, Y. (2019). Determination of total inorganic arsenic in water samples by cadmium ion assisted photochemical vapor generation-atomic fluorescence spectrometry. Microchemical Journal, 146, 359-365. https://www.sciencedirect.com/science/article/pii/S0026265X18318320
 write
write