Abstract
Donor cell leukemia (DCL) is a rare but severe complication identified in 1971 in a female patient who developed acute lymphoblastic leukemia (ALL) 62 days post-transplantation from her brother. Cytogenic analysis showed XY karyotype wove pointing toward a donor cell origin of leukemia(Fialkow et al.,1971). The condition usually arises after allogeneic stem cell transplantation (SCT), a curative modality for haematological malignancies. Patients undergoing SCT treatment have recurring leukemia; up to 5% are DCT(Williams et al., 2021). There has been no clear cause of DCL. However, alteration of the microenvironment during SCT may increase the likelihood that the progenitor cells from the host become leukemia. The proposed mechanism for the development of DCL includes and is not limited to the presence of an underlying stromal defect either inherent in the host or those acquired during radiotherapy and conditioning regiments, the transformation of donor cells by viral or host antigenic stimulation, oncogene transection by fusion of donor cells with the residues of leukemia cells, presence of occult leukemia in the donor among others(Engel et al., 2019). Recent advancements in molecular chimerism monitoring have indicated a rising upsurge in such cases of DCL. However, they could have been there before and only realised due to advancements in diagnostic techniques. The identification of the DCL and the risk factors associated with it is critical in the identification of the donors as well as potential concerns on the health of the donors, and ethical consideration in notifying the donor of future possibilities of emergence of leukemia, particularly in case of umbilical cord blood transplantation which is among the risk factors of DCL.
Introduction
Allogeneic hematopoietic stem cell (HSCT) is a treatment modality for haematological malignancies, particularly acute myelogenous leukemia. However, disease relapse remains a significant challenge (Adachi Y.,2018).DCL is a new form of leukemia that develops in donor-derived haematopoetic precursor cells and whose incidence remains underestimated due to poor prognosis to distinguish between recipient-derived and donor-derived cells post HCCT. However, current genetic sequencing techniques have an allergy to differentiation. Such methods include fluorescence in situ hybridization (FISH) that detects and locates specific DNA sequences. DCL is commonly diagnosed with a median time of 31 months (Wiseman, DH.,2011).
Table 1:Main difference between original leukemia and donor cell leukemia according to WHO classification.
| Element | Original leukemia | Donor cell leukemia |
| Myeloperoxidase stain | Positive | Negative |
| Cell surface markers | Positive: CD13, CD34, HLA-DR
Negative: CD7,CD33,CD41,CD56 |
Positive: CD7, CD13, CD21,CD34,CD33,CD38,CD41,CD56,HLA-DR |
| G-banding stain | 47, XY, +1, der(1;7)
(q10;p10),+8[13]/46,XY[7] |
46,XX[20] |
| WHO classification | AML with myelodysplasia-related changes | Acute megakaryoblastic leukemia |
Although there have been no notable causes of DCL, there have been progressive steps in determining the risk factors associated with DCL. A range of possible risk factors includes the age of the donor and the source of the stem cell, which could either be bone marrow, umbilical cord blood, or perspiration blood.
Other causes are conditioning therapy and intensity. Such factors have been associated with the ability to influence the microenvironment of the bone marrow, which ultimately influences the genomic stability of the donor’s stem cell.
i) Source of stem cell
Umbilical cord blood, peripheral blood, and bone marrow are the progenitor cells used in HSCT. It has been noted that the source of stem cells used influences the chances of DCL occurring, the duration of time between the transplantation and the occurrence of DCL, and the types of karyotypes(Shiozaki H.,2014).
Umbilical cord blood(UCB) is a feasible source of progenitor cells for allo-HSCT patients, particularly those lacking HLA matcher bone marrow donors. UCB had been identified to be the risk factor for DCL. Despite being the most used type of stem cell source, UCB has been linked to acute myeloid leukemia (AML) cases. Systemic screening of a large series of unselected cord blood samples revealed that approximately 1% harboured putative pre-leukemia mic clones with the AML-1 fusion gene. Using such ‘pre-leukemia’ samples as a source of stem cells might involve a risk of DCL(Ando T et al., 2006).
In addition, cord blood cells are immunologically naive. Their naivety makes them more tolerant of a tissue mismatch between the donor’s cord blood cells and the recipient.
The characteristics of DCL occurring after umbilical cord blood transplantation differed from those that occurred when a donor is transplanted with a bone marrow transplant, including the time between transplantation and the occurrence of DCL. Donor cells occurred within a shorter period in DCL. The median duration for DCL to occur after transplantation with UCB is 14.5 months, and 36 months for BMT(Shiozaki H., 2014)
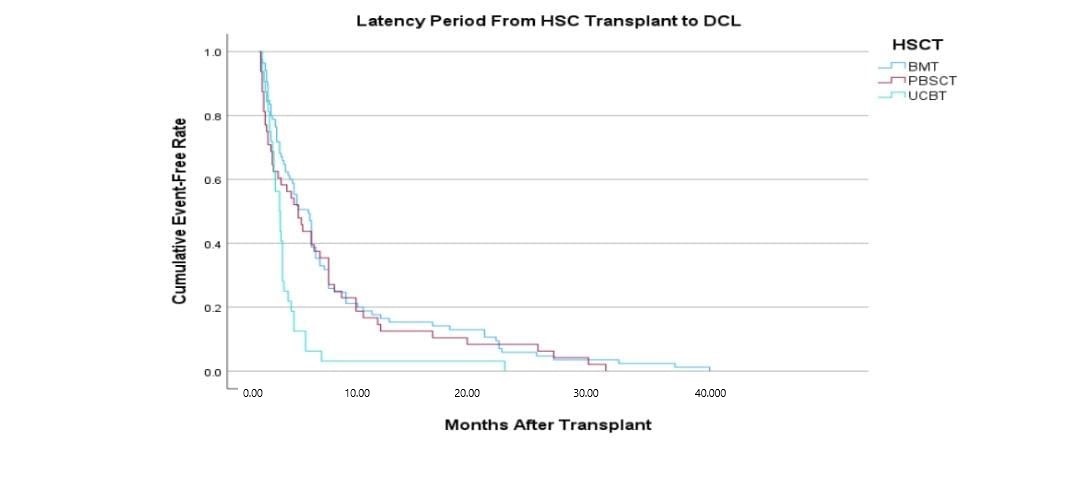
Figure 1: The latency time taken between HSCT to development or DCL.UCB shows a bigger risk for the development of DCL.
ii) Chemotherapeutic agents
a) Alkylating agents
Alkylating agents are chemotherapeutic drugs used against malignant cells and leukemia and lymphoma patients. Common samples of those drugs include cyclophosphamide, carmustine, Meghalaya, and busulfan. These drugs interfere with the cells’ DNA transcription—the drugs act by cross-linking strands of DNA, specifically at the N7 position of the base guanine. Exposure of the genome to the alkylating agents interferes with the chemical composition of the DNA. As such, under rare circumstances, a misrepair of the DNA through methyl adducts and cross-link formation result in genotoxic outcomes, and high exposure to doses of alkylating agents, DNA repair may result in increased mutation(Klapacz et al., 2016).
Chemotherapy using these alkylating agents causes cellular damage to mesenchymal stromal cells(MSC) in vitro and invivo(May J., Morse, 2012). If there is damage to the MSC, these two may result in graft failure, and the damage induced in the DNA may increase the risk of genetic instability and, thus, malignancies develop (Popp & Bohlander.,2000).
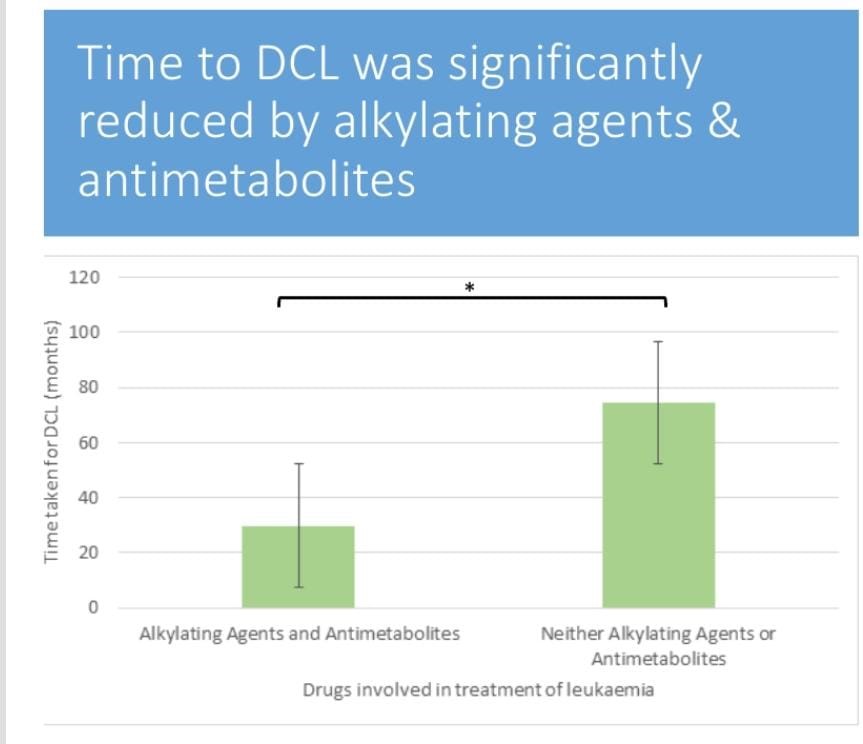
Figure 2: The time taken for DCL to be diagnosed reduced among patients who have been on chemotherapeutic drugs of class.
Alkylating agents are significantly reduced compared to the time the DCL takes to be diagnosed in patients on other drugs, which are neither alkylating agents nor antimetabolies.
b) Non-myeloablative(NMA)versus myeloablative(MA).
NMA regimens use less doses of total body imitation and may cause mild myelosuppression and low treatment toxicity.MA uses high doses and requires stem cell support(Balkan M.,2017). MA, which is highly toxic, is expected to cause DCL faster than NMA. However, the NMA genotoxicity is enough to cause DCL.
c) Bystander radiation damage
DCL leukemogenesis has been linked with bystander radiation damage through several mechanisms. First, the oncogenic materials released by the cell during radiation possibly transfer into the donor’s HSCT and cause damage(Havelange et al.,2006)
iii) Leukomogenic marrow environment
The bone marrow microenvironment maintains the HSCT’s Homeostasis (Duarte D., 2018). Alteration of mesenchymal stem cells, stromal cells, osteoblasts, and adipocytes have been shown to cause the initiation of leukemia and its progression and development of therapy-resistant leukemia clones(Witkowski MT., 2020).
Therefore, if such leukemia and transformed bone marrows have their cells defective, replacing such bone marrows through allo-HCT may not be sufficient to prevent donor cell leukemia.
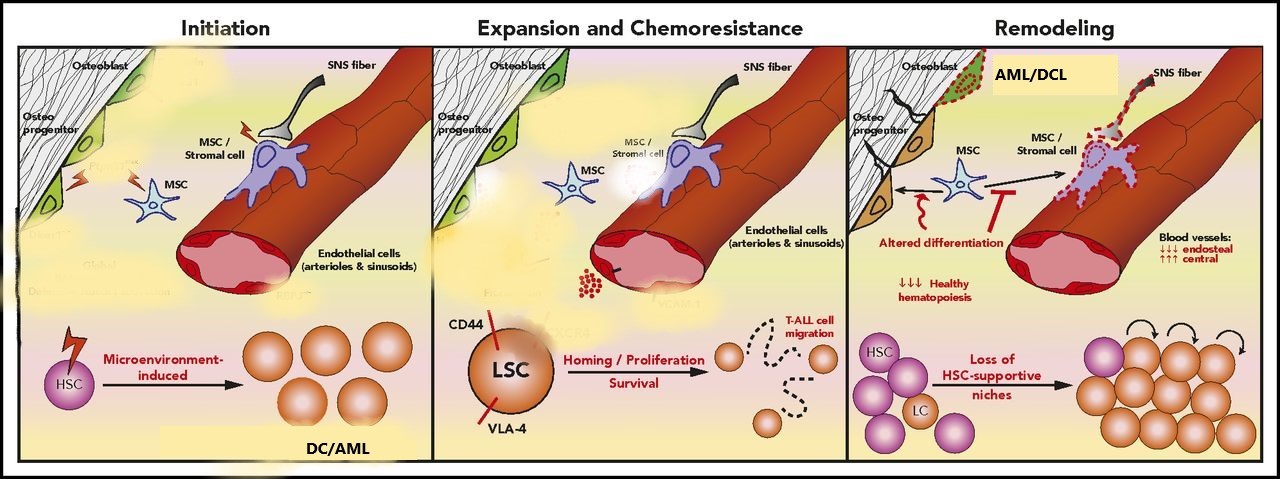
Figure 3: The cross-link between leukemia cells and the microenvironment.
The impaction stage involves alterations of signalling pathways in specific cell types in osteoblastic and stroma cells. The expansion and chemoresistance state involves the leukemic stem cell(LSC) interacting with the microenvironment and proliferating using adhesive molecules(CD44 and VLA-4). As the HSC proliferates (remodeling), it impairs the MSC differentiation and destroys HSC-supportive hinches. This results in recipient-donor cell-derived leukemia; DCL melody plastic syndrome.
Conclusion
DCL remains a rare complication whose prognosis is not well defined, but possible risk factors have been discussed on how they could affect its aetiology. While the pathogenesis of DCL remains complex, the improvement in detection, genetic analysis, and detailed screening of the donors of the stem cells for germline mutations and history of malignancy remains and could offer greater insight into the detection and prevention of DCL post-HSCT.
References
Adachi Y, Yamaguchi Y, Sagou K, Yamaga Y, Fukushima N, Ozeki K, Kohno A. Acute Megakaryoblastic Leukemia Developing as Donor Cell Leukemia after Umbilical Cord Blood Transplantation. Intern Med. 2018 Feb 15;57(4):569-574. doi: 10.2169/internalmedicine.9005-17. Epub 2017 Nov 20. PMID: 29151503; PMCID: PMC5849555.
Aldoss I, Song JY, Curtin PT, Forman SJ. Multiple donor-derived leukemias in a recipient of allogeneic hematopoietic cell transplantation for myeloid malignancy. Blood Adv. 2020 Oct 13;4(19):4798-4801. doi 10.1182/bloodadvances.2020002803. PMID: 33022063; PMCID: PMC7556138.
Ando T, Yujiri T, Mitani N, Takeuchi H, Nomiyama J, Suguchi M, Matsubara A, Tanizawa Y. Donor cell-derived acute myeloid leukemia after unrelated umbilical cord blood transplantation. Leukemia. 2006 Apr;20(4):744-5. doi: 10.1038/sj.leu.2404121. PMID: 16437136.
Engel N, Rovo A, Badoglio M, Labopin M, Basak GW, Beguin Y, Guyotat D, Ljungman P, Nagler A, Schattenberg A, Schroeder T, Schroyens W, Tischer J, Socie G, Kolb HJ, Tichelli A, Salooja N, Duarte RF; Transplant Complications Working Party of the European Society for Blood and Marrow Transplantation. European experience and risk factor analysis of donor cell-derived leukemias/MDS following haematopoietic cell transplantation. Leukemia. 2019 Feb;33(2):508-517. doi: 10.1038/s41375-018-0218-6. Epub 2018 Jul 26. PMID: 30050122.
Fialkow PJ, Thomas ED, Bryant JI, Neiman PE. Leukaemic transformation of engrafted human marrow cells in vivo. Lancet. 1971
Havelange V, Antoine-Poirel H, Saussoy P, Van Den Neste E, Ferrant A. Donor cell leukemia developing after hematopoietic stem cell transplantation for multiple myeloma. Acta Clin Belg. 2006 Mar-Apr;61(2):82-6. doi: 10.1179/acb.2006.016. PMID: 16792340.
Jennifer E May, Craig Donaldson, Liana Gynn, H Ruth Morse, Chemotherapy-induced genotoxic damage to bone marrow cells: long-term implications, Mutagenesis, Volume 33, Issue 3, May 2018, Pages 241–251
Klapacz J, Pottenger LH, Engelward BP, Heinen CD, Johnson GE, Clewell RA, Carmichael PL, Adeleye Y, Andersen ME. Contributions of DNA repair and damage response pathways to the non-linear genotoxic responses of alkylating agents. Mutat Res Rev Mutat Res. 2016 Jan-Mar;767:77-91. doi: 10.1016/j.mrrev.2015.11.001. Epub 2015 Dec 2. PMID: 27036068; PMCID: PMC4818947.
Popp, H. D. and Bohlander, S. K. (2010). Genetic instability in inherited and sporadic leukemias. Genes. Chromosomes Cancer, 49, 1071–1081.
Williams L, Doucette K, Karp JE, Lai C. Genetics of donor cell leukemia in acute myelogenous leukemia and myelodysplastic syndrome. Bone Marrow Transplant. 2021 Jul;56(7):1535-1549. doi: 10.1038/s41409-021-01214-z. Epub 2021 Mar 8. PMID: 33686252.
Wiseman DH. Donor cell leukemia: a review. Biol Blood Marrow Transplant 17: 771-789, 2011.
 write
write