Introduction
In chemistry, an acid is described as a substance that, in a water solution, tastes sour. Acid can also be described as any substance that donates hydrogen ions (H+) and gains electrons during a reaction (Gurbel et al., 2023). The pH of an acid is below 7, which can be used to indicate its strength. An acid can be categorized into strong/weak acids. An acid is defined as strong when it has a high concentration of hydrogen ions (H+). A weak acid has a low concentration of hydrogen ions (H+). Other classifications of acid include monoprotic and polyprotic acids. A monoprotic acid is described as a type of acid that donates a single hydrogen atom per molecule of an aqueous solution (Helmenstine, 2022). Consider the equation below;
HA (aq) +H20 (l) H30+ (aq) + A– (aq)
Some examples of monoprotic acids include all acids with single hydrogen atoms, such as Nitric acids and hydrochloric acids. On the other side, polyprotic acids donate more than one hydrogen atom when they dissociate. A diprotic acid donates two hydrogen atoms when they dissociate. Some examples of polyprotic acids include sulfuric acids (H2SO4) and carbonic acids (H3 SO4).
A base is described as a substance that can neutralize an acid and has a pH of above 7. The base is also described as a substance that accepts (OH–). Some examples of bases include sodium hydroxide and alkali metals (Marie, 2019). Alkali is a chemical substance that can dissolve in water and combine with acids to form salts. Alkali has a bitter taste, and they turn certain dyes blue (Clark, 2020).
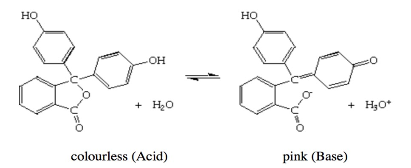
Titration is a laboratory method that is used to determine the concentration of a given analyte. The process of titration involves the preparation of a standard solution with known concentration and volume, which is called titrant. During the process of titration, an indicator will be used. An indicator is described as a chemical compound that changes its initial color in the presence of either acid or base. An indicator (phenolphthalein) will be used to determine the exact end-point for the titration. The phenolphthalein indicator is colorless in acid (initial color) and pink in acid. During titration of acid (HCL) and base (sodium hydroxide) neutralization reaction, the acid will neutralize when the color of the solution changes to pink, as indicated by the image below;
Aim
The aim of this exploration was to use the titration method to determine the concentration of two unknown acids (A and B). The concentration of two unknown acids will be determined by titrating sodium hydroxide with a known concentration (0.1). The following formula will later be used to find the concentration of both acids;
Where;
= concentration of sodium hydroxide
= volume of sodium hydroxide (average volume)
= concentration of unknown (Acid A)
= volume of sodium of unknown (Acid B)
Materials and methods
For this experiment, all students were requested to wear lab coats, and other safety clothing such as spectacles and gloves throughout this experiment. The acid is very corrosive and irritant to the skin, and thus, safety precautions should be considered.
- The burette was rinsed with sodium hydroxide before filing it with the same solution. A filter funnel was used to fill the burette with sodium hydroxide.
- The initial recording of the burette was recorded to the nearest 0.5 mL, as indicated in the results table below.
- By the use of a pipette, 20 mL of Acid A was measured and transferred into a conical flask. A dropper was used to add 3 drops of phenolphthalein indicator into the flask containing acid A.
- The sodium hydroxide from the burette was added into the conical flask drop by drop, and the color changed to pink (the color of the phenolphthalein indicator in an alkaline solution), confirming the endpoint.
- The volume of sodium hydroxide used to neutralize (Acid A) was recorded in Table 1 below.
- All the contents from this experiment were discarded in the sink with running water.
- By the use of a pipette, 20 mL of unknown Acid A was measured and transferred into a conical flask. Three drops of phenolphthalein indicator were also added to the flask.
- Sodium hydroxide was added to the mixture while swirling until the volume added was 1 mL less than the endpoint, which was obtained in step (4).
- When the endpoint was achieved, sodium hydroxide was added drop by drop while checking for color change (colorless to pink). Record the volume of sodium hydroxide used in the result table below.
- Steps 4-9 were repeated while using a fresh sample of Acid A and recording the volume of sodium hydroxide required to neutralize the acid.
- Steps 2-10 were repeated while using the second unknown acid (B) by adding 20 mL of the cad to the conical flask and recording the rough volume and two accurate volumes in the table below.
- The burette was emptied of sodium hydroxide and filled with distilled water.
- Determine the concentration of Acid A and Acid B
Results and calculations
Table 1: results table
| Name of Acid
|
Titration | Initial volume reading (mL) | Final volume reading (mL) | The volume of 0.1 M sodium hydroxide required to neutralize acid |
| Acid A | Rough | 0.0 | 19.1 | 19.1 |
| Accurate#1 | 19.1 | 38.3 | 19.3 | |
| Accurate#2 | 0.0 | 19.1 | 19.1 | |
| Acid B | Rough | 0.0 | 20 | 20.0 |
| Accurate#1 | 20.0 | 39.7 | 19.7 | |
| Accurate#2 | 0.0 | 19.8 | 19.8 |
Average volume of (NaOH): Acid A
To find the average volume of sodium hydroxide,
Calculation for unknown acid (A) concentration
To find the concentration of acid (A), the following process will be followed;
Where;
= concentration of sodium hydroxide
= volume of sodium hydroxide (average volume)
= concentration of unknown (Acid A)
= volume of sodium of unknown (Acid A)
The concentration of unknown Acid (A) was 0.0960 M.
Calculation for unknown acid (B) concentration
To find the concentration of acid (B), the following process will be followed;
Where;
= concentration of sodium hydroxide
= volume of sodium hydroxide (average volume)
= concentration of unknown (Acid B)
= volume of sodium of unknown (Acid B)
The concentration of unknown Acid (B) was 0.0988 M.
Questions
Question 1:
Given that the formula to find pH is;
Given that the pH of 0.1 M HCL is 1 and the pH of 0.1 M Acetic Acid is 2.88, determine the H+ of each acid.
Different acids have different amounts of (H+), as indicated by the computation above. Acids have different hydrogen ions since different acids have different strengths. Strong acids such as HCL (pH 1) dissociate completely, and they produce a high concentration of hydrogen ions (H+). Weak acids such as acetic acids (pH 2.88) dissociate partly, thus producing a low concentration of hydrogen ions (H+)
Question 2: if you titrate the same volume of 0.1 M HCL and 0.1 acetic acids against sodium hydroxide, both acids will require the same volume of sodium hydroxide to neutralize the acid. Explain
Both weak and strong acids (HCL and Acetic Acid) will require the same volume of sodium hydroxide to neutralize this because strong acid dissociates completely in water. When we add sodium hydroxide, the concentration of (H+) reduces rapidly. Although weak acids dissociate less in water when sodium hydroxide is added to the weak acids, the acid starts producing hydrogen ions rapidly, and thus, it will require the same volume to neutralize.
Discussion
Through titration, it was possible to compute the concentration of each acid since the volume and concentration of sodium hydroxide used were known in both cases. The concentration of Acid A was identified to be 0.0960 M, while the concentration of Acid B was 0.0988, indicating that both acids have almost the same concentration. Based on the results above, therefore, it is not possible to determine whether the acid is a weak or strong acid. It was later identified that Acid A was HCL, which is a strong acid. At the same time, Acid B was (Acetic Acid), which is a weak acid. In this experiment, it was clear that both acids have the same theoretical concentration (0.1) and different hydrogen concentrations. However, the same volume of sodium hydroxide was used to neutralize the acid.
Errors and uncertainties
The experiment was a huge success as the aim of the experiment was achieved. However, no experiment is perfect, and thus, it is vital to determine the error in this experiment. To find the error in this experiment, the following formula will be applied;
Acid A
The theoretical concentration= 0.1000 (HCL)
While finding the concentration of Acid A, there was 4% which is a significant error.
Acid B
The theoretical concentration= 0.1000 (Acetic Acid)
While finding the concentration of Acid A, there was 1.2% which is a significant error.
Based on the percentage errors above, it was evident that there were some mistakes which might lead to some errors. The concentration of sodium hydroxide was only given from the stock solution, and it might be incorrect. It is, therefore, important to prepare the solution in the lab before the experiment to increase accuracy.
Conclusion
The aim of this exploration was to use the titration method to determine the concentration of two unknown acids (A and B). This was possible by titrating the acids with sodium hydroxide with a known concentration (0.1M). The average volume of sodium hydroxide used in titration was used to find the concentration of both acids. A phenolphthalein indicator was used to mark the endpoint for this experiment. The concentration of Acid A was (0.0960 M while the concentration of Acid B was 0.0988) and thus, the aim of this experiment has been achieved. Although the experiment was a success, the % error for finding the concentration of acids A and B was 4.0% and 1.2%, respectively. A high percentage indicates that there were some errors and mistakes during the experiment.
References
Clark, J. (2020, October 3). Group 1: Properties of Alkali Metals. Chemistry LibreTexts. https://chem.libretexts.org/Bookshelves/Inorganic_Chemistry/Supplemental_Modules_and_Websites_(Inorganic_Chemistry)/Descriptive_Chemistry/Elements_Organized_by_Block/1_s-Block_Elements/Group__1%3A_The_Alkali_Metals/1Group_1%3A_Physical_Properties_of_Alkali_Metals
Gurbel, P. A., Bliden, K. P., Kundan, P., Kraft, D., Parekh, R., Singh, S., & Tantry, U. S. (2023). Early assessment of the pharmacokinetic and pharmacodynamic effects following acetylsalicylic acid loading: toward a definition for acute therapeutic response. Journal of Thrombosis and Thrombolysis, 1-8.
Helmenstine, A. (2022, May 31). Monoprotic Acid Definition and Examples. Science Notes and Projects. https://sciencenotes.org/monoprotic-acid-definition-and-examples/
Marie, A. (2019). What Is a Base in Chemistry? ThoughtCo. https://www.thoughtco.com/definition-of-base-604382
 write
write