Hazards associated with ethylene oxide
Ethylene oxide (EO), a highly reactive, combustible, and explosive gas, is frequently used to produce various consumer goods, including pharmaceuticals, food, and medical equipment. It is, however, a very poisonous material that endangers both the environment and human health (Jones et al.,2023). In this thorough investigation, we will look at the dangers of ethylene oxide and any potential concerns to human health and the environment.
The toxicity of ethylene oxide is one of the main risks it poses. The potent alkylating chemical EO can interact with proteins, DNA, and other biological components, resulting in cellular damage and disruption (Kirman et al.,2021). The harmful health effects of EO exposure can include cancer, central nervous system depression, irritated skin and eyes, irritated respiratory system, and skin and eye irritation. According to studies, long-term exposure to EO has been linked to an increased risk of leukaemia, lymphoma, and other cancers.
Ethylene oxide also possesses flammability and explosive properties. When exposed to heat or an open flame, the highly reactive gas, EO, can ignite and explode. It poses a severe fire risk and explosion, particularly in industrial settings where it is frequently utilized in huge quantities (Liu et al.,2021). The handling and storage of EO must be done correctly to reduce the risk of fire and explosion.
Ethylene oxide can lead to air pollution and groundwater contamination, which are environmental risks. An air pollutant called EO, or volatile organic compound, can combine with other air pollutants like nitrogen oxides to create ground-level ozone, which is hazardous to human health and the environment (Jones et al.,2023). Moreover, incorrect ethylene oxide disposal can contaminate soil and groundwater, which can have long-lasting repercussions on regional ecosystems.
Finally, it should be noted that ethylene oxide is a highly poisonous, combustible, and explosive gas that poses severe risks to human health and the environment. Due to its high flammability and explosiveness, EO poses a severe fire and explosion risk and several other harmful health effects, including cancer. Procedures for handling, storing, and disposing of ethylene oxide must be appropriately followed to reduce dangers. To ensure that EO is utilized safely and ethically, monitoring and regulating its use is crucial.
Examples of incidents that illustrate these types of hazards
These occurrences highlight the significant dangers of ethylene oxide, including its explosiveness, flammability, and toxicity. They also emphasize the significance of handling, storing, and disposing of ethylene oxide properly to avoid accidents, safeguard environmental safety, and safeguard human health.
Facility owned by Sterigenics in Willowbrook, Illinois: In 2018, people who lived close to the Sterigenics facility in Willowbrook, Illinois, learned that the business had been releasing ethylene oxide into the atmosphere for years. According to the EPA, ethylene oxide is a recognized human carcinogen, and the facility’s emissions were determined to be 30 times above the allowable level (Jones et al.,2023). In 2019, the facility was finally shut down, and the business was required to make a $200 million compensation payment to the locals whom the pollutants had harmed.
The Bhopal tragedy occurred in 1984 when a chemical leak at the Union Carbide facility in Bhopal, India, caused a cloud of hazardous chemicals, including ethylene oxide, to be discharged into the neighbourhood (Liu et al.,2021). The catastrophe quickly claimed the lives of nearly 3,000 people and had long-term health impacts on hundreds of thousands more. One of the disaster’s significant aspects was the discharge of ethylene oxide.
Incident at the Tosoh Bioscience plant in King of Prussia, Pennsylvania: In 2004, an ethylene oxide explosion at the Tosoh Bioscience facility in King of Prussia, Pennsylvania, resulted in the death of a worker (Kirman et al.,2021). When a spark ignited the ethylene oxide while the worker was transferring it from one tank to another, an explosion resulted in the worker’s death and the injury of numerous others.
Oxygen balance equation calculation for the compounds: Ammonium nitrate, Trinitrotoluene, Benzene, and water
In studying explosive chemistry, the oxygen balance equation is a helpful tool for calculating the amount of oxygen needed for a molecule to burn totally. A chemical with a positive oxygen balance has more oxygen than is necessary, while one with a negative one has less oxygen than necessary. This analysis will compute the oxygen balance equation for four substances—ammonium nitrate, Trinitrotoluene, benzene, and water—(Kumar & Elias,2019). The findings will be compared and discussed.
Predicting how various molecules will burn is made more accessible by using oxygen balance. Understanding the stability, flammability, and reactivity of different compounds by calculating oxygen balance can help create safer and more effective chemical processes (Kumar & Elias,2019). Remembering that oxygen balance is merely a theoretical idea and might not always precisely represent how various substances behave in the real world is crucial.
Oxygen balance = (total number of oxygen atoms in the molecule) – (total number of hydrogen atoms in the molecule/2) – (total number of carbon atoms in the molecule/4) – (total number of nitrogen atoms in the molecule/2)
For Ammonium Nitrate (NH4NO3): Total number of oxygen atoms = 3 Total number of hydrogen atoms = 4 Total number of nitrogen atoms = 1 Total number of carbon atoms = 0
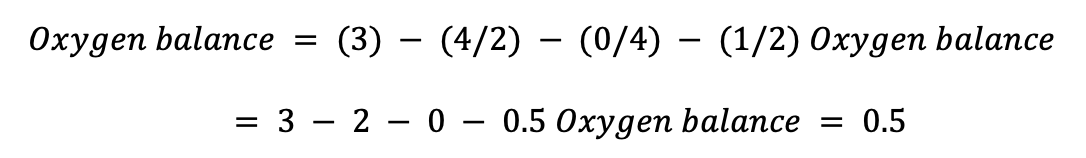
For Trinitrotoluene (C7H5N3O6): Total number of oxygen atoms = 6 Total number of hydrogen atoms = 5 Total number of nitrogen atoms = 3 Total number of carbon atoms = 7

For Benzene (C6H6): Total number of oxygen atoms = 0 Total number of hydrogen atoms = 6 Total number of nitrogen atoms = 0 Total number of carbon atoms = 6
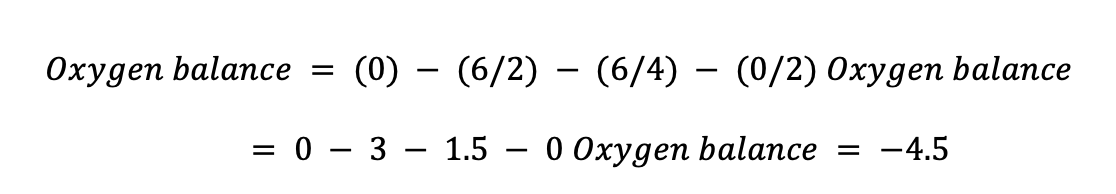
For Water (H2O): Total number of oxygen atoms = 1 Total number of hydrogen atoms = 2 Total number of nitrogen atoms = 0 Total number of carbon atoms = 0
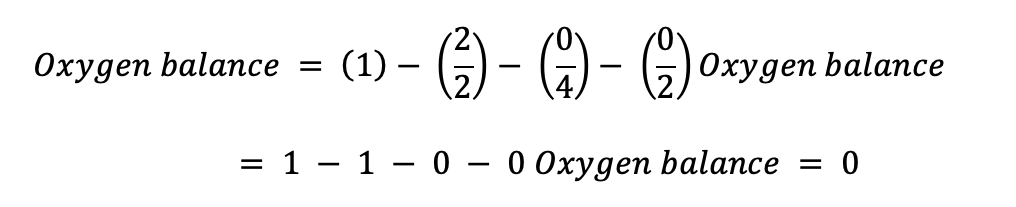
For each of the four molecules—ammonium nitrate (NH4NO3), Trinitrotoluene (C7H5N3O6), benzene (C6H6), and water—we have determined the oxygen balance (H2O). Ammonium nitrate has an oxygen balance of 0.5, meaning that it has a little deficiency in oxygen atoms and needs an oxidizing agent to undergo complete combustion. On the other hand, Trinitrotoluene has a 0.25 oxygen balance, which similarly implies an oxygen atom deficiency, albeit to a lesser amount than ammonium nitrate (Sultan et a.,2022). Trinitrotoluene is more stable than ammonium nitrate and less likely to burn spontaneously.
The relatively stable and inert chemical benzene has an interestingly negative oxygen balance of -4.5. This finding suggests that benzene can spontaneously burn without the aid of an oxidizing agent (Sultan et al.,2022). Nonetheless, benzene is often utilized as a solvent rather than fuel or explosive chemical and is not considered a highly flammable substance in everyday life.
Last, water has an oxygen balance of 0, indicating that it is a fully balanced molecule and does not require oxidizing or reducing chemicals for combustion. Our concept of water as a stable, inert component that does not readily interact with other compounds is consistent with this result.
Alternative theoretical methods that may provide better results than the OB methods
A theoretical approach for determining a material’s explosibility is the oxygen balance (OB). The foundation of this technique is that for something to burn or explode, there needs to be enough oxygen to sustain the combustion process. The OB is computed by deducting the total amount of oxygen atoms from the weighted average of the molecules’ Hydrogen, carbon, and nitrogen atom counts (Gibot et al.,2020). The obtained number is used to forecast the material’s possible explosibility.
Explosive materials research and development have made substantial use of the OB method. It is a commonly used and trustworthy approach for determining possible risks and estimating the explosibility of novel substances (Gibot et al.,2020). By altering the proportion of oxidizing agents in the mixture to obtain a desired OB value, the approach has also been used to optimize the formulation of explosives.
Unfortunately, the OB approach has some drawbacks that may reduce the precision with which it can predict explosibility. Its assumption that every oxygen atom in the molecule will take part in burning is one of its limitations. However, this is only sometimes the case. For instance, the oxygen might occasionally be chemically bonded and not readily available for combustion. The OB technique also ignores other elements that might influence explosibility, like the material’s susceptibility to friction or shock.
Other theoretical approaches have been proposed to solve these drawbacks and may offer superior outcomes than the OB method. The heat of explosion (HOE) method is one such approach based on the quantity of heat emitted during combustion (Yin Wang & Lu,2019). The HOE approach thoroughly evaluates a material’s explosibility, accounting for the energy needed to break chemical bonds and the energy released during burning.
Another approach is the critical oxygen index (COI), based on the lowest amount of oxygen needed in a mixture of the material and air to support burning (Yin Wang & Lu,2019). This technique accurately indicates a material’s explosiveness under particular circumstances, such as temperature and oxygen content.
The OB method is a well-known and trustworthy theoretical technique for determining a material’s explosibility. Its shortcomings, however, may make it less accurate at foretelling explosibility. Other theoretical approaches, such as the HOE and COI methodologies, have been put forth and may offer superior outcomes by considering additional explosibility-affecting elements (Lin et al.,2021). The particular needs of the application and the properties of the material being assessed will determine the approach to be used.
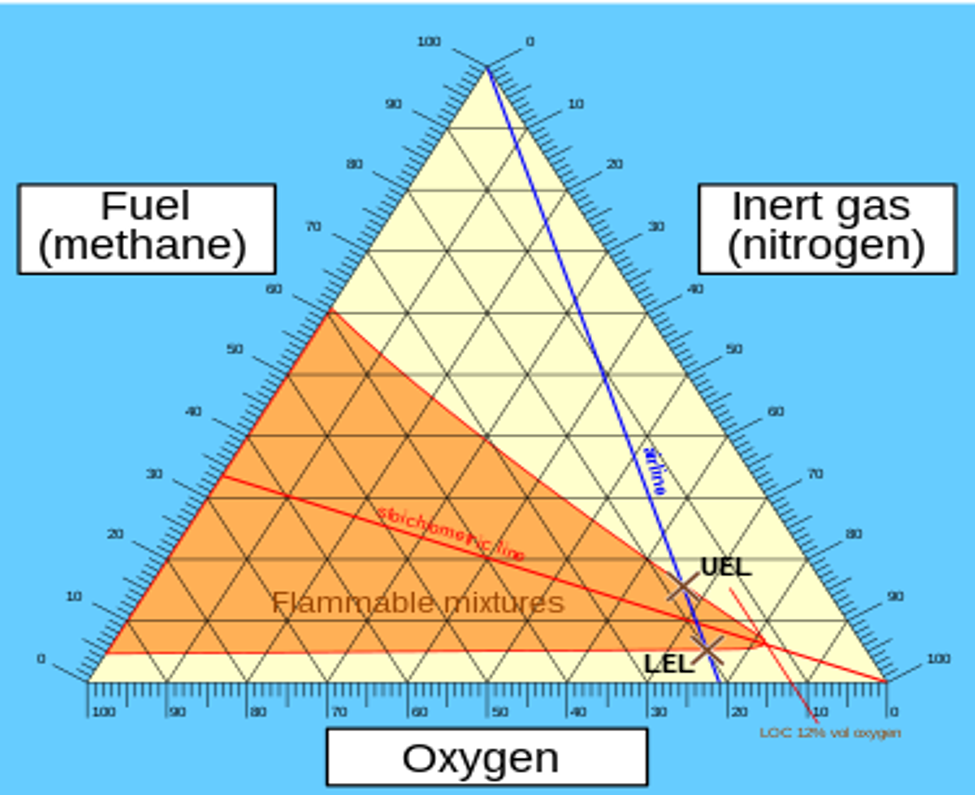
Flammability diagram of carbon monoxide (CO) and Hydrogen
TNT equivalency method Solve the problems from Crowl & Louvar 6.9 and 6.14
The TNT equivalency method is frequently used to compare the explosive power of various materials to that of TNT, which is regarded as a standard reference material. The approach is predicated on the idea that the energy produced by a material of a particular weight when it detonates is proportionate to the energy released by a substance of a weight equal to TNT (Stennett, Gaulter & Akhavan,2020). The relative explosive strength of material concerning TNT is expressed using the TNT equivalency factor (TEF).
The mass of the substance being considered is divided by the mass of TNT necessary to release the equivalent amount of energy to determine the TEF. The TEF of a material would be 1.0, for instance, if it exploded with the same amount of energy as 1 kg of TNT. A material’s TEF would be 0.5 if it needed twice as much TNT to discharge the same amount of energy (Stennett, Gaulter & Akhavan,2020). To calculate the blast overpressure and damage brought on by an explosion using a particular substance, utilize the TNT equivalency approach. Regardless of the energy source, the technique assumes that an explosion’s effects are proportionate to the overall quantity of energy released.
Calculate the TNT equivalent factor for propane given that the heat of explosion for propane is 46.9 MJ/kg and the heat of explosion for TNT is 4.18 MJ/kg in Crowl & Louvar’s book “Chemical Process Safety,” problem 6.9. Applying the TEF formula, we obtain.
Hence, propane has a TEF of 11.2, which indicates that it possesses an explosive force 11.2 times greater than TNT. Calculate the weight of ANFO (ammonium nitrate-fuel oil) needed to provide the same amount of energy as 100 kg of TNT, according to problem 6.14 from the same book. ANFO explodes at a heat of 3.7 MJ/kg, while TNT explodes at a heat of 4.18 MJ/kg. We can use the TEF calculation to get the weight of ANFO needed:
Hence, in order to generate 100 kg of energy, 88.5 kg of ANFO would be needed.
References
Gibot, P., Vidal, L., Laffont, L., & Mory, J. (2020). Zirconia nanopowder synthesis via detonation of Trinitrotoluene. Ceramics International, 46(17), 27057-27062. https://www.sciencedirect.com/science/article/pii/S0272884220321957
Jones, R. R., Fisher, J. A., Medgyesi, D. N., Buller, I. D., Liao, L. M., Gierach, G., … & Silverman, D. T. (2023). Ethylene oxide emissions and incident breast cancer and non-Hodgkin lymphoma in a US cohort. JNCI: Journal of the National Cancer Institute, djad004. https://academic.oup.com/jnci/advance-article-abstract/doi/10.1093/jnci/djad004/6986131
Kirman, C. R., Li, A. A., Sheehan, P. J., Bus, J. S., Lewis, R. C., & Hays, S. M. (2021). Ethylene oxide review: characterization of total exposure via endogenous and exogenous pathways and their implications for risk assessment and management. Journal of Toxicology and Environmental Health, Part B, 24(1), 1–29. https://www.tandfonline.com/doi/abs/10.1080/10937404.2020.1852988
Kumar, D., & Elias, A. J. (2019). The Explosive Chemistry of Nitrogen: A Fascinating Journey From 9th Century to the Present. Resonance, 24, 1253-1271. https://link.springer.com/article/10.1007/s12045-019-0893-2
Lin, W., Shao, Y., Li, G., Guo, Y., & Zhan, X. (2021). The psychological implications of COVID-19 on employee job insecurity and its consequences: The mitigating role of organization adaptive practices. Journal of Applied Psychology, 106(3), 317. https://psycnet.apa.org/record/2021-37196-001
Liu, Y., Zong, L., Zhang, C., Liu, W., Fakhri, A., & Gupta, V. K. (2021). Design and structural of Sm-doped SbFeO3 nanopowders and immobilized on poly (ethylene oxide) for efficient photocatalysis and hydrogen generation under visible light irradiation. Surfaces and Interfaces, p. 26, 101292. https://www.sciencedirect.com/science/article/pii/S2468023021003692
Stennett, C., Gaulter, S., & Akhavan, J. (2020). An estimate of the Beirut port explosion’s TNT‐equivalent net explosive quantity (NEQ) using publicly‐available tools and data. Propellants, Explosives, Pyrotechnics, 45(11), 1675-1679. https://onlinelibrary.wiley.com/doi/abs/10.1002/prep.202000227
Sultan, M., Wu, J., Haq, I. U., Imran, M., Yang, L., Wu, J., … & Chen, L. (2022). Recent Progress on Synthesis, Characterization, and Performance of Energetic Cocrystals: A Review. Molecules, 27(15), 4775. https://www.mdpi.com/1420-3049/27/15/4775
Yin, Y., Wang, Y., & Lu, Y. (2019). Antecedents and outcomes of employee empowerment practices: A theoretical extension with empirical evidence. Human Resource Management Journal, 29(4), 564-584. https://onlinelibrary.wiley.com/doi/abs/10.1111/1748-8583.12243
 write
write