Introduction
The HPV vaccine, or the Human Papilla Movirus vaccine, is important in preventing cervical cancer and other types associated with this group of viruses. Cervical cancer is a serious global issue that affects women around the world, ranking as the fourth most common and second most prevalent in the United States. Studies have shown a definite link between HPV infections and cervical malignancies, particularly with high-risk strains of the virus (Seyferth et al., 2016). Unfortunately, underserved areas face a greater burden of this type of cancer due to limited access to diagnostic testing and proactive measures.|
The advent of HPV vaccination technology has marked a major milestone in the fight against cervical malignancies. The vaccine strategy zeroes in on the most dangerous viruses linked to cancer, dramatically lowering the chance of cervical cancer via pre-emptive action against early viral infections (Krokidi et al., 2023). Moreover, progress in HPV screening technology, such as incorporating HPV tests into cervical cancer screening programs, has markedly improved the accuracy of identifying high-risk cervical cell abnormalities (CIN) and early-stage cervical cancer.
Studies on the efficacy and epidemiology of HPV vaccine-associated cervical cancer outcomes are important for the following reasons:
Cervical cancer prevention: Cervical cancer is largely preventable through early detection and HPV vaccination. Vaccines are most effective when given before exposure to the virus, usually before sexual activity begins (Vichnin et al., 2015).
Public Health Impact: HPV-related cancers, including cervical cancer, have a significant impact on public health systems, causing a substantial burden of disease and healthcare costs (Aimagambetova et al., 2022). Researching the efficacy of the HPV vaccine allows healthcare providers and policymakers to make informed decisions about the inclusion of the vaccine in national immunization programs, which can lead to a reduction in the incidence of cervical cancer and associated healthcare expenditures.
Targeted Vaccination Programs: By understanding the effectiveness of the HPV vaccine, healthcare authorities can design targeted vaccination programs that reach populations at the highest risk of HPV infection and cervical cancer (Zhou et al., 2022). These programs might include specific age groups, communities with limited access to healthcare, and regions with a higher prevalence of HPV-related diseases.
Long-term Impact: Researching the long-term effects of the HPV vaccine on reducing cervical cancer rates is essential for ensuring the sustained success of vaccination efforts. Monitoring the incidence of cervical cancer over time in vaccinated populations can help identify any potential waning immunity and guide decisions about booster doses or updates to the vaccine formulation (Landier et al., 2022).
Aim
The primary aim of this literature review is to comprehensively assess and analyse the impact of two key interventions, the Human Papillomavirus (HPV) vaccine and HPV testing, on the epidemiology of cervical cancer. This review seeks to elucidate the effectiveness of these interventions in altering the incidence, prevalence, and burden of cervical cancer on a population level.
Objectives
- To evaluate the effectiveness of the HPV vaccine in reducing HPV-related outcomes, including cervical intraepithelial neoplasia (CIN) and, ultimately, cervical cancer.
- To evaluate the effect of HPV vaccines on HPV infection rates and their subsequent impact on cervical cancer incidence.
- Investigate the long-term effects of HPV vaccination by analysing population-based studies in different age groups and cohorts.
- Explore the role of HPV testing, especially co-testing, in improving the accuracy of cervical cancer screening by improving sensitivity and specificity.
- To examine the potential of HPV testing to detect high-grade CIN and prevent progression to invasive cervical cancer.
- To synthesize the evidence on the combined effects of the HPV vaccine and HPV screening, consider how these interventions reduce cervical cancer morbidity and mortality.
- Identify challenges, gaps, and future research directions in cervical cancer prevention strategies, focusing on coverage of HPV vaccines and advances in screening techniques.
Methods
The literature searches have been conducted using defined search strategies in peer-reviewed sources. The clinical search was focused on identifying published studies related to HPV vaccination and its impact on the occurrence of cancer. Various databases were searched extensively, including Medline, PubMed, Scopus, Cochrane Database, Science Direct, Embase, and Review Summary Database (DARE). This review aimed to gather data regarding the effectiveness of HPV vaccination and explore how it influences cancer epidemiology. We specifically aimed to enhance our understanding of the vaccine’s efficacy and examine the prevalence of cancer within populations. The search strategies involved using controlled vocabulary like topic titles and keywords from the National Library of Medicine (MeSH). Key concepts covered in this study included HPV testing, cervical cancer screening methods, diagnosis approaches for cancer, evaluation of ATDs (Adverse Treatment Effects), and screening methods.
Numerous studies have showcased the effectiveness of HPV vaccines in reducing infection rates caused by the viruses they target (Skufca et al., 2017). The remarkable findings from prominent trials conducted in Costa Rica and known as FUTURE II demonstrate a significant decrease in HPV-related outcomes, such as CIN and genital warts, particularly among those vaccinated (J. Wang et al., 2019). These studies prove that vaccines can effectively prevent the early stages of disease progression linked to HPV. Additionally, population-based investigations have revealed lower cervical precancerous lesions in teenagers who received the HPV vaccine early, yielding impressive results (Oliveira & Niccolai, 2021). These findings underscore the long-term potential of vaccines to reshape the trajectory of cervical cancer development.|
Moreover, the integration of HPV testing and conventional cytology-based screening, so-called simultaneous testing, has significantly improved the sensitivity and specificity of abnormality detection in the cervix. This approach is important for rapidly identifying high-grade CIN, allowing rapid intervention to prevent progression to invasive cervical cancer. In the realm of cervical cancer screening, institutions like the Cochrane Collaboration have consistently highlighted the capacity of HPV testing to enhance accuracy (Suzuki & Hosono, 2018). By reducing the occurrence of invasive cervical cancer, HPV testing proves itself as a substantial breakthrough in prevention tactics against this disease.
The Inclusion and Exclusion Criteria
Studies satisfying the specified criteria have been assessed and are represented in this review (Maver & Poljak, 2018). The criteria for inclusion consisted of assessing the vaccine’s ability to prevent cervical cancer and reviewing studies that provided insight into its impact on epidemiological trends and the benefits it confers to HPV screening. A blend of RCTs and observational research was analysed to strike an equilibrium between varying study designs. Studies from introducing the HPV vaccine to the present are included to capture a global perspective.
Data Mining and Synthesis
The systematic collection of data from each chosen study forms the foundation of the extraction process. Key aspects include the study design parameters, participant profile, vaccine specifications (type and dosage), outcome measures (HPV infection rate, CIN incidence, cervical cancer incidence), and the impact of HPV vaccination and screening (Skufca et al., 2017). Combining the obtained information enables a thorough grasp of the distinct effects of these interventions on the population dynamics of cervical cancer.
Quality Evaluation
A quality check was implemented to confirm the validity of the evidence collated. Variables, including a research plan, participant pool, technique, and potential pitfalls, were evaluated in this assessment (J. Wang et al., 2019). The objective is to pinpoint every investigation’s strengths and weaknesses and integrate the findings into the broader evaluation landscape.
PICO Study Design
Population
The population selected for this study included women at risk for HPV and cervical cancer. It includes people of all ages, from teenagers to the elderly (Yuan et al., 2022). Age does not limit susceptibility to HPV infection because sexual activity can lead to viral infection. In addition, cervical cancer risk is influenced by long-term cumulative exposure to high-risk HPV types. This approach requires a comprehensive analysis of the impact of HPV vaccines and testing methods at different life stages.
Intervention
Interventions of interest include the administration of HPV vaccines that target specific oncogenic viruses, primarily HPV-16 and HPV-18. These vaccines work by inducing an immune response against viral antigens, thereby preventing persistent infection by the target virus (Pei et al., 2023). This specific HPV type was chosen because it is closely linked with the majority of cervical cancer cases.
Comparison
Comparative groups of unvaccinated individuals or populations with limited coverage are essential to assess the efficacy of HPV vaccines. This comparison helps determine the direct contribution of vaccines to reducing HPV-related outcomes (Joura et al., 2015). In an endeavour to single out the impacts of vaccination interventions, this research aimed to explore the dissimilarities in HPV rates, CIN prevalence, and cervical cancer cases among both vaccinated and non-vaccinated groups.
Outcome
The main interesting results revolve around three interrelated indicators: HPV prevalence, CIN incidence, and cervical cancer incidence. The decline in HPV infection rates reflects the vaccine’s ability to limit early viral infection and prevent the spontaneous progression of cervical cancer (Avian et al., 2022). The reduced incidence of CIN, especially high-grade lesions, reflects the vaccine’s efficacy in preventing the development of precancerous abnormalities of the cervix. Ultimately, the decline in cervical cancer incidence marks the culmination of vaccine efficacy in preventing the most severe manifestations of HPV-related disease.
Results
In the study conducted by Goodman et al. (2022), they explored existing literature on PubMed and Medline databases, uncovering a vast collection of 2,200 records. To further enhance their review process, an additional 16 records were included for analysis (Figure 1). The researchers utilized the PICO standard in a two-step process: initially rejecting 2,137 applications after evaluating their titles and abstracts; then eliminating another 49 applications following a thorough assessment of their full texts (Baisley et al., 2022). Eventually, only 30 applications remained eligible for consideration.
While vaccine efficacy studies surpassed vaccine effectiveness studies in number significantly. Recurrent respiratory papillomatosis patients were the primary focus of investigation concerning vaccine efficacy (12 studies), followed by women suffering from HPV-associated genital disease. Several studies have analyzed the efficacy of vaccines in different populations, including men who have sex with men (MSM) and individuals with compromised immunity. Nevertheless, transgender and non-binary communities, as well as sex workers, were not specifically studied (Kim et al., 2022). It is worth noting that a few MSM studies did include a limited number of transgender women in their analyses.
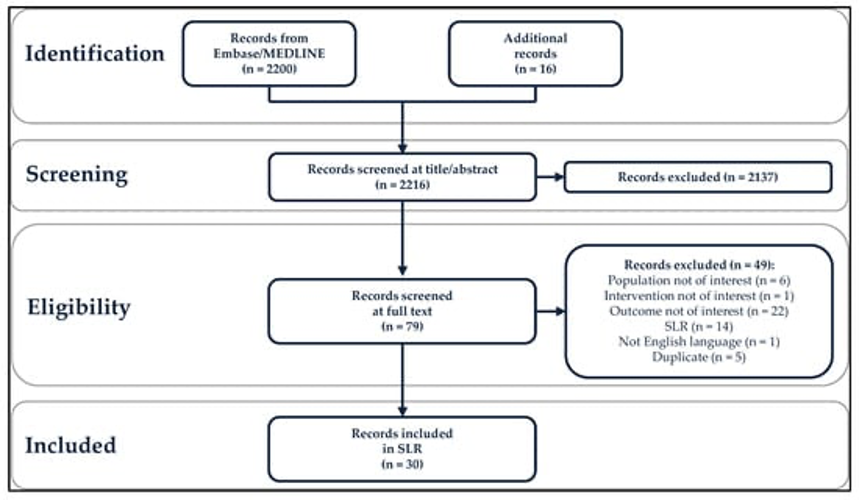
Figure 1. Preferred Reporting Items for Systematic Reviews and Meta-Analyses Diagram (PRISMA). SLR, systematic literature search (Goodman et al., 2022).
In a clinical study conducted by Chao et al. (2019), they report the findings of thorough research that involved examining a total of 7,128 citations gathered through an extensive literature search. Out of these citations, 170 were deemed potentially relevant systematic reviews (SRs) along with three SRs found within the text itself. The team then proceeded to assess the relevance of these 173 titles and abstracts concerning their review objectives. After evaluating the title and abstract content, a smaller subset consisting of 19 full-text publications was selected for further analysis following the PICO criteria established by Stuebs et al. (2022). Unfortunately, fifteen SRs had to be excluded due to various reasons; however, four SRs ultimately matched all inclusion criteria and were consequently incorporated into this comprehensive review.
After conducting a review, it was discovered that the existing systematic review (SR) falls short of addressing all the pertinent outcomes related to the research query. Consequently, final decisions regarding inclusion within the SR are made based on individual findings. Four separate SRs incorporated in their reports outcomes about the diagnostic effectiveness of HPV primary screening with or without cytological classification when compared to cytology-based primary screening for cancer detection.
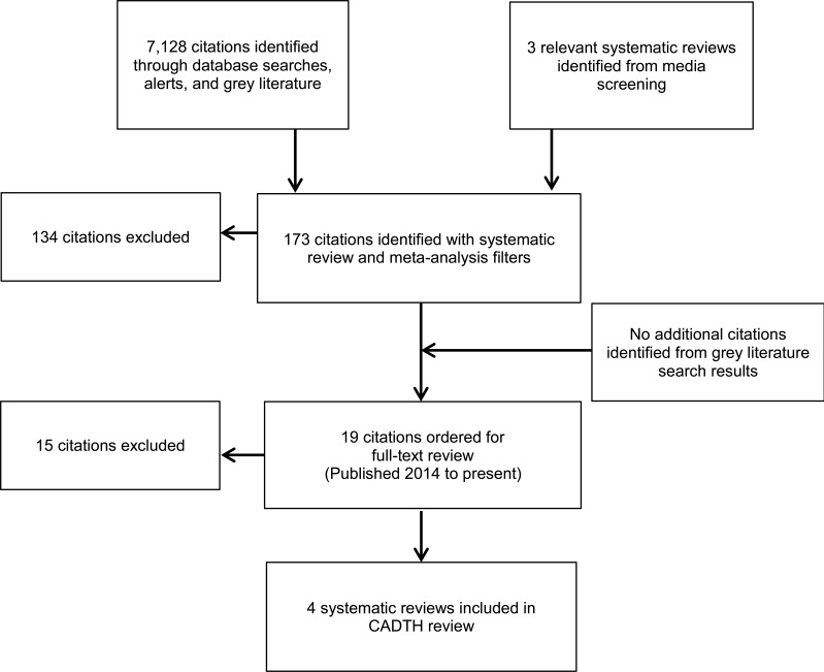
Figure 2: A flow diagram illustrating the literature selection process for SRs (Chao et al., 2019).
In a systematic review conducted by Ortiz et al. (2019), various databases were searched to gather relevant information for the study. The search results included 145 citations from PubMed, 107 citations from Sociological Summary, 47 citations from PsycINFO, and additional citations from other sources such as media outlets (11), Elite Business Source (13), and ERIC (13). In total, 325 articles were identified. After an initial screening of titles and abstracts, it was found that out of the 301 unique citation results obtained, some articles were redundant.
Upon conducting a thorough examination of each selected paper’s full-text content, only forty-four papers were deemed appropriate for inclusion in the study (as shown in Figure 3). Among these chosen articles, considerable focus was placed on analysing social media interactions and content. Specific social media platforms like Facebook (n=12), Twitter (n=10), and YouTube(n=8) received significant attention along with less popular ones like Google+, Myspace, and Instagram, as well as comment sections and discussion forums across various online social network platforms with five instances combined. The remaining content comprises 15 articles, which delve into the realm of data collection from individuals. Most researchers aim to examine how exposure to this content can shape people’s knowledge and attitudes towards HPV vaccination.
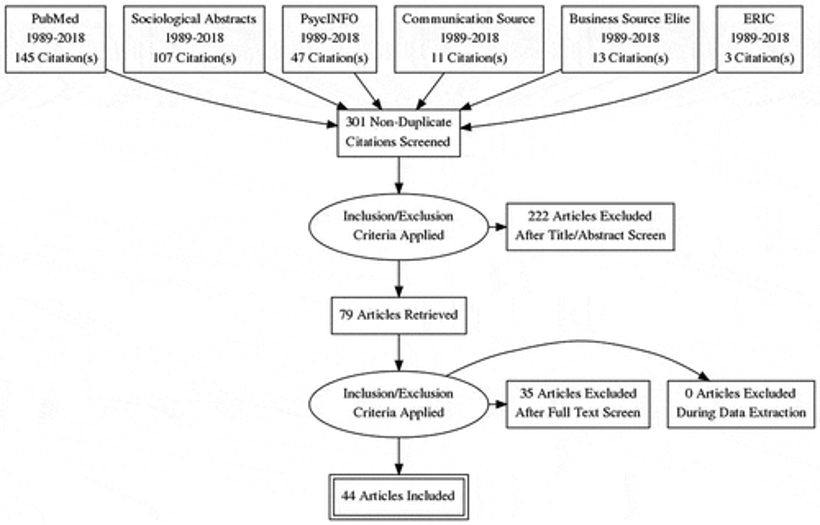
Figure 3: PRISMA diagram (Ortiz et al., 2019).
Research by Krokidi et al. (2023) gave results showing that, between the years 1975 and 2019, a total of 63,304 new cases of cervical cancer were identified in individuals aged 20-44. Out of these cases, there were 42,220 (66.69%) carcinomas and other histological subtypes classified as cervical squamous cell carcinoma (SCC), along with 13,694 (21.63%) cases of cervical adenocarcinoma (AC) and 2,456 (3.88%) cases of other types. Additionally, 4,934 (7.79%) cases could not be grouped into a specific subtype. Incidence rates for all types of cervical cancer in individuals aged 20-44 declined over time (-0.7% overall per year). The incidence rate for SCC specifically decreased even further at -1.0% per year when excluding the periods between 1980-1994 or 2010-2019. The frequency of cervical CA has consistently risen, growing to 2.6% per annum from 1975 to 2019 (Aasbø et al., 2022). In terms of cervical cancer and cervical SCC combined, the surge between 2010 and 2019 was predominantly observed among individuals aged 30-34, 35-39, and 40-44.
Based on the research findings, it was observed that there has been a consistent pattern regarding cervical cancer rates in individuals between the ages of 20-24 and 25-29. However, a significant increase in incidence was noted among older age groups. Birth cohort trends were also analysed for specific age groups about the occurrence of cervical cancer and squamous cell carcinoma. On the other hand, while the incidence of cervical CA remained steady in individuals aged 20-24 years old and experienced an overall decrease in those aged 30-44 years old and those aged 25-29 years old recorded a notable rise. Analysing data from individuals born between 1983 and 1997 revealed a decline in incidence specifically within the age groups spanning from 20 to 24 years old as well as those ranging from ages 25 to 19.
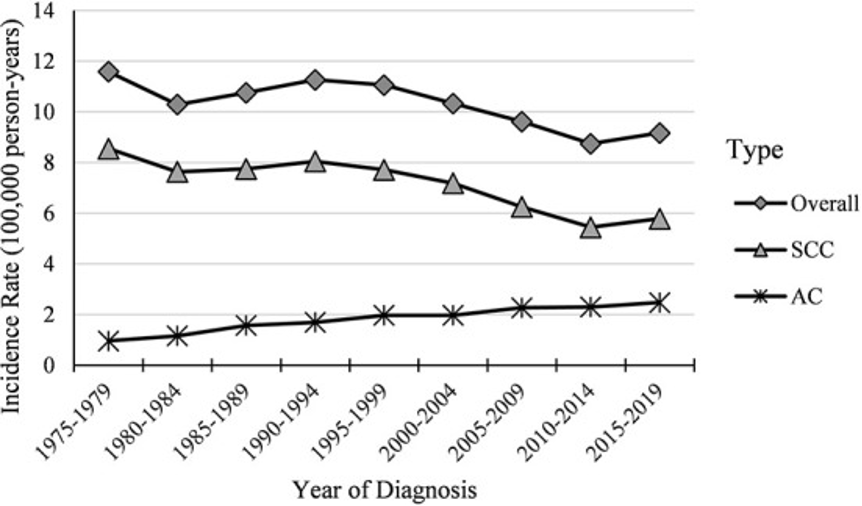
Figure 4: Temporal trends in cervical cancer incidence among women aged 20-44 years (1975-2019) (Krokidi et al., 2023).
Discussion
In the research conducted by Goodman et al. (2022), the effectiveness of the 4vHPV and 9vHPV vaccines in preventing HPV-related diseases has been demonstrated through clinical trials, showing long-lasting protection. To further strengthen our understanding of these vaccines’ efficacy and effectiveness, empirical research has been conducted by evaluating their impact on primary and re-compensation groups. The evidence collected shows strong and sustained protective benefits. Despite the availability of previously published studies on this topic, certain high-risk populations have not been adequately represented or studied extensively. These populations are significant as they either fall under national immunization programs or are being considered for such support. To address this knowledge gap, a comprehensive review of related literature aims to compile and analyse data specifically focused on the actual effects and outcomes of using 4vHPV/9vHPV vaccines within six high-risk populations.
This finding is consistent with previous reviews that have assessed the efficacy of HPV vaccines in preventing the recurrence of cervical cancer and RRP (W. Wang et al., 2022). Most studies have focused on the 4vHPV vaccine, which has been around for eight years longer than the 9vHPV vaccine, indicating that it will take time for actual impact and efficacy studies to emerge. Given this circumstance, such a result is expected.
Furthermore, there remains a significant dearth of research regarding the real-life impact and effectiveness of HPV vaccines against specific outcomes related to HPV-related diseases across different populations (Godoy et al., 2022). None of the studies have examined how effective or impactful these vaccines are against head, neck, penile, or vaginal cancers. These knowledge gaps underscore the need for further investigation to understand their potential benefits fully. Limited evidence exists regarding the effectiveness and efficiency of vaccines in populations such as MSM, individuals living with HIV, and those with weakened immune systems. Likewise, there is a lack of specific vaccine efficacy or influence on transgender individuals, non-bisexual persons, and sex workers, highlighting the necessity for further understanding regarding these vulnerable groups.
After surpassing the literature search threshold set for the included SRs, 21 primary studies led to the discovery of twenty-two relevant publications in the clinical review by Chao et al. (2019). Findings from Cochrane and HIQA reviews were examined to analyse diagnostic test accuracy (DTA). The author of the Cochrane review directly compared three HPV tests (HC2, Aptima, and PCR [which encompasses over thirteen viral strains]) with cytology. Meanwhile, the HIQA review compared HC2 with cytology. Both reviews reached similar conclusions regarding HPV thresholds equal to or higher than one pg/mL or 1 RLU:
– HC2 offers greater sensitivity in detecting CIN2+ and CIN3+ when compared against ASCUS threshold cytology.
– Conversely, HC2 exhibits less specificity when identifying CIN2+ and CIN3+.
A Cochrane review discovered that the accuracy of HPV testing in predicting CIN3+ varied depending on the level of validation bias in the studies. When focusing exclusively on CIN3+, studies involving individuals aged 30 years and above reported a higher sensitivity for HPV testing compared to those including all appropriate screening ages (93.9% [CI up to 95%, with a range from 89.3% to 96.6%] versus 92.6% [95% CI, ranging from 89.6% to 95.3%]). It aligns with expectations since participants aged 30 years and older have higher-grade lesions. Of the eight primary studies reviewed after the publication of SR, seven found that HPV tests (HC2, Multiplex Genotype, Aptima, Cobas, Confidence, etc.) are more sensitive than LBC and conventional cytologic tests, It was concluded that the specificity was low.
In a study conducted by Jin et al., it was discovered that HC2 exhibits greater sensitivity and specificity than cytology. The inconsistent findings of this research lack a definitive explanation. Nevertheless, the authors failed to mention the specific diagnostic threshold employed for the HC2 test and did not account for potential bias when analysing their results. SR evaluated individuals’ responses to screening invitations. The evaluation drew upon an overview of findings compiled by Bennett et al. (2015). Based on estimates, procedure, and intent-to-treat analyses imply the potential of sending her collection for HPV testing through the mail. All participants had access to electricity. Being late for screening is more lenient than regular cervical cancer screening. The acceptability of self-sampled HPV testing options, regardless of the approach analyzed, demonstrated no substantial disparities compared to traditional cervical cancer screening methods. It is evident that whether individuals were offered door-to-door screenings or presented with self-collected HPV test kits due to delays in their regular screening routine, there was negligible variation observed in acceptance rates when contrasted with conventional practices.
The findings of the assessment from the systematic literature review by Ortiz et al. (2019) reveal that social media can offer a wealth of information and exchange regarding HPV and its vaccination. However, it should be noted that the information available may not always be comprehensive, accurate, or substantiated. Unfavourable content concerning vaccine efficacy and protection is prevalent on social media platforms, often stemming from sources lacking medical credibility. This spread of misinformation surrounding HPV vaccination has raised apprehension among parents and healthcare practitioners about its potential negative impact on the overall acceptance rate of the vaccine.
Exposure to distressing material on social media has been associated with the persistent rejection of the HPV vaccine, causing a decrease in HPV vaccination rates. It also allows for more opportunities to disseminate unpleasant content among users on social networking sites. Some organizations have expressed additional concerns about coverage of HPV vaccinations, and mainstream news coverage on this topic tends to fuel ongoing discussions on social media platforms. While positive content often manages to reach a wide audience, negative content can easily be shared by users within closely-knit communities online. Anti-vaccine narratives frequently raise worries regarding protection efficacy, potential side effects, and overall effectiveness of vaccines. Conversely, pro-vaccination materials commonly emphasize its benefits in safeguarding oneself rather than focusing much on its protective ability or efficiency.
To effectively engage individuals who are leaning towards vaccination for their well-being and protection, it can be beneficial for fitness professionals and communicators to create social media content specifically tailored to address their concerns. This type of content may include providing additional information that elaborates on vaccine efficacy and safety aspects (Kechagias et al., 2022). Comparatively, anti-vaccination content tends to lack credibility, often omitting hyperlinks or references pointing toward trusted medical sources. Consequently, such content struggles to reach a wider audience or establish meaningful connections with them. When crafting messages aimed at these audiences, fitness experts and broadcasters should exercise caution regarding their choice of profiles. These target individuals may not wholeheartedly accept viewpoints presented by established fitness bodies or institutions if they have previously embraced conspiracy theories involving authorities and pharmaceutical companies. Maintaining awareness about this potential scepticism among the audience becomes crucial when communicating important information about vaccinations.
The review encompasses numerous studies that reveal significant engagement and curiosity among individuals regarding accurate and beneficial information about HPV and HPV vaccines through social media (Ortiz et al., 2019). It becomes especially crucial for those who access sexual health-related information online, exhibiting hesitance towards discussing such matters in person; a phenomenon believed to be influenced, at least partially, by the implications associated with sexually transmitted infections. Interacting through social media platforms allows individuals to exercise greater control over their flow of information and maintain a higher degree of confidentiality when participating in conversations compared to traditional face-to-face group discussions.
A comprehensive analysis conducted by Krokidi et al. (2023) explores the prevalence of cervical cancer in the United States, considering its repercussions and potential shifts amongst diverse time frames and age brackets. Concrete evidence affirms that HPV vaccines effectively mitigate the risk of developing cervical cancer, regardless of its histologic classification. Numerous variables play a role in modifying the occurrence rate of this malignant condition. Some reasons can include exposure to diethylstilbestrol while still in the womb, obesity, and long-term usage of hormonal contraceptives. However, two factors are primarily attributed to a rise in overall cervical cancer cases. According to the 5th Edition, WHO Classification of Female Genital Tumours, a significant majority (over 90-95%) of cervical squamous cell carcinomas arise due to high-risk HPV genotypes; meanwhile, there is also considerable importance given to HPV-associated cervical partial adenocarcinoma (Rosenblum et al., 2022).
HPV 16 and 18 play a prominent role in contributing to almost all cases of HPV-associated cervical adenocarcinoma. As an HPV infection triggers most cervical cancers, administering an HPV vaccine decreases its occurrence. This administration may also be attributed to the influence exerted by current guidelines for cervical cancer screening. In particular, from the 1960s through the 1990s, the Papanicolaou test (Pap) and Simpleprep Cytology (TCT) were widely employed. However, subsequent developments incorporated within screening protocols include utilizing either a Pap/HPV DNA co-test or primary test since 2012 and 2018, respectively. The ability of the Pap and TST tests to detect cervical squamous cell carcinoma is higher compared to detecting adenocarcinoma (Lee et al., 2022). However, limited knowledge regarding the natural course of cervical adenocarcinoma makes it uncertain how much impact Pap testing has on the associated morbidity. In the United States, between 1975 and 2013, there was a similar episodic increase in incidence rates for cervical adenocarcinoma as seen in other HPV-related cancers that are not detected through screening, such as nasopharyngeal, oropharyngeal, and anal cancer. Therefore, examining the trends in incidence rates and cohort effects related to cervical adenocarcinoma over time may better reflect the influence of HPV vaccination efforts.
References
Aasbø, G., Tropè, A., Nygård, M., Christiansen, I. K., Baasland, I., Iversen, G. A., Munk, A. C., Christiansen, M. H., Presthus, G. K., & Undem, K. (2022). HPV self-sampling among long-term non-attenders to cervical cancer screening in Norway: A pragmatic randomised controlled trial. British Journal of Cancer, 127(10), 1816–1826.
Aimagambetova, G., Babi, A., Issa, T., & Issanov, A. (2022). What factors are associated with attitudes towards HPV vaccination among Kazakhstani women? Exploratory analysis of cross-sectional survey data. Vaccines, 10(5), 824.
Avian, A., Clemente, N., Mauro, E., Isidoro, E., Di Napoli, M., Dudine, S., Del Fabro, A., Morini, S., Perin, T., & Giudici, F. (2022). Clinical validation of full HR-HPV genotyping HPV Selfy assay according to the international guidelines for HPV test requirements for cervical cancer screening on clinician-collected and self-collected samples. Journal of Translational Medicine, 20(1), 1–12.
Baisley, K., Kemp, T. J., Kreimer, A. R., Basu, P., Changalucha, J., Hildesheim, A., Porras, C., Whitworth, H., Herrero, R., & Lacey, C. J. (2022). Comparing one dose of HPV vaccine in girls aged 9–14 years in Tanzania (DoRIS) with one dose of HPV vaccine in historical cohorts: An immunobridging analysis of a randomised controlled trial. The Lancet Global Health, 10(10), e1485–e1493.
Bennett, A. T., Patel, D. A., Carlos, R. C., Zochowski, M. K., Pennewell, S. M., Chi, A. M., & Dalton, V. K. (2015). Human papillomavirus vaccine uptake after a tailored, online educational intervention for female university students: A randomized controlled trial. Journal of Women’s Health, 24(11), 950–957.
Chao, D. Y.-S., Clark, M., Carson, E., Weeks, D. L., Moulton, K., McFaul, D. S., McLauchlin, D. C. M., Tsoi, D. B., Majid, U., Kandasamy, S., Arora, N., Vanstone, D. M., Reid, D. L., Krahn, T., Kaluzny, K., Farrah, K., & Ford, C. (2019). Clinical Review. In HPV Testing for Primary Cervical Cancer Screening: A Health Technology Assessment [Internet]. Canadian Agency for Drugs and Technologies in Health. https://www.ncbi.nlm.nih.gov/books/NBK543096/
Godoy, L. R., Possati-Resende, J. C., Guimarães, Y. M., Pedrão, P. G., Dos Reis, R., & Longatto-Filho, A. (2022). Implementation of HPV tests in Latin America: What we learned; what should we have learned, and what can we do better? Cancers, 14(11), 2612.
Goodman, E., Reuschenbach, M., Kaminski, A., & Ronnebaum, S. (2022). Human Papillomavirus Vaccine Impact and Effectiveness in Six High-Risk Populations: A Systematic Literature Review. Vaccines, 10(9), Article 9. https://doi.org/10.3390/vaccines10091543
Joura, E. A., Giuliano, A. R., Iversen, O.-E., Bouchard, C., Mao, C., Mehlsen, J., Moreira Jr, E. D., Ngan, Y., Petersen, L. K., & Lazcano-Ponce, E. (2015). A 9-valent HPV vaccine against infection and intraepithelial neoplasia in women. New England Journal of Medicine, 372(8), 711–723.
Kechagias, K. S., Kalliala, I., Bowden, S. J., Athanasiou, A., Paraskevaidi, M., Paraskevaidis, E., Dillner, J., Nieminen, P., Strander, B., & Sasieni, P. (2022). Role of human papillomavirus (HPV) vaccination on HPV infection and recurrence of HPV related disease after local surgical treatment: Systematic review and meta-analysis. Bmj, 378.
Kim, S. J., Schiffelbein, J. E., Imset, I., & Olson, A. L. (2022). Countering Antivax misinformation via social media: Message-testing randomized experiment for human papillomavirus vaccination uptake. Journal of Medical Internet Research, 24(11), e37559.
Krokidi, E., Rao, A. P., Ambrosino, E., & Thomas, P. P. M. (2023). The impact of health education interventions on HPV vaccination uptake, awareness, and acceptance among people under 30 years old in India: A literature review with systematic search. Frontiers in Reproductive Health, 5, 1151179. https://doi.org/10.3389/frph.2023.1151179
Landier, W., Bhatia, S., Wong, F. L., York, J. M., Flynn, J. S., Henneberg, H. M., Singh, P., Adams, K., Wasilewski-Masker, K., & Cherven, B. (2022). Immunogenicity and safety of the human papillomavirus vaccine in young survivors of cancer in the USA: A single-arm, open-label, phase 2, non-inferiority trial. The Lancet Child & Adolescent Health, 6(1), 38–48.
Lee, J.-E., Chung, Y., Rhee, S., & Kim, T.-H. (2022). Untold story of human cervical cancers: HPV-negative cervical cancer. BMB Reports, 55(9), 429.
Maver, P. J., & Poljak, M. (2018). Progress in prophylactic human papillomavirus (HPV) vaccination in 2016: A literature review. Vaccine, 36(36), 5416–5423.
Oliveira, C. R., & Niccolai, L. M. (2021). Monitoring HPV vaccine impact on cervical disease: Status and future directions for the era of cervical cancer elimination. Preventive Medicine, 144, 106363.
Ortiz, R. R., Smith, A., & Coyne-Beasley, T. (2019). A systematic literature review to examine the potential for social media to impact HPV vaccine uptake and awareness, knowledge, and attitudes about HPV and HPV vaccination. Human Vaccines & Immunotherapeutics, 15(7–8), 1465–1475. https://doi.org/10.1080/21645515.2019.1581543
Pei, J., Shu, T., Wu, C., Li, M., Xu, M., Jiang, M., & Zhu, C. (2023). Impact of human papillomavirus vaccine on cervical cancer epidemic: Evidence from the surveillance, epidemiology, and end results program. Frontiers in Public Health, 10, 998174. https://doi.org/10.3389/fpubh.2022.998174
Rosenblum, H. G., Lewis, R. M., Gargano, J. W., Querec, T. D., Unger, E. R., & Markowitz, L. E. (2022). Human papillomavirus vaccine impact and effectiveness through 12 years after vaccine introduction in the United States, 2003 to 2018. Annals of Internal Medicine, 175(7), 918–926.
Seyferth, E., Bratic, J., & Bocchini, J. (2016). Human papillomavirus epidemiology and vaccine recommendations: Selected review of the recent literature. Current Opinion in Pediatrics, 28. https://doi.org/10.1097/MOP.0000000000000354
Skufca, J., Ollgren, J., Ruokokoski, E., Lyytikäinen, O., & Nohynek, H. (2017). Incidence rates of Guillain Barré (GBS), chronic fatigue/systemic exertion intolerance disease (CFS/SEID) and postural orthostatic tachycardia syndrome (POTS) prior to introduction of human papilloma virus (HPV) vaccination among adolescent girls in Finland, 2002–2012. Papillomavirus Research, 3, 91–96.
Stuebs, F. A., Koch, M. C., Dietl, A. K., Adler, W., Geppert, C., Hartmann, A., Knöll, A., Beckmann, M. W., Mehlhorn, G., & Schulmeyer, C. E. (2022). Cytology and high-risk human papillomavirus test for cervical cancer screening assessment. Diagnostics, 12(7), 1748.
Suzuki, S., & Hosono, A. (2018). No association between HPV vaccine and reported post-vaccination symptoms in Japanese young women: Results of the Nagoya study. Papillomavirus Research, 5, 96–103.
Vichnin, M., Bonanni, P., Klein, N. P., Garland, S. M., Block, S. L., Kjaer, S. K., Sings, H. L., Perez, G., Haupt, R. M., & Saah, A. J. (2015). An overview of quadrivalent human papillomavirus vaccine safety: 2006 to 2015. The Pediatric Infectious Disease Journal, 34(9), 983–991.
Wang, J., Ploner, A., Sparén, P., Lepp, T., Roth, A., Arnheim-Dahlström, L., & Sundström, K. (2019). Mode of HPV vaccination delivery and equity in vaccine uptake: A nationwide cohort study. Preventive Medicine, 120, 26–33.
Wang, W., Kothari, S., Skufca, J., Giuliano, A. R., Sundström, K., Nygård, M., Koro, C., Baay, M., Verstraeten, T., & Luxembourg, A. (2022). Real-world impact and effectiveness of the quadrivalent HPV vaccine: An updated systematic literature review. Expert Review of Vaccines, 21(12), 1799–1817.
Yuan, J., Xie, W., Lan, G., Li, X., & Zhu, X. (2022). Research on the current situation and coping strategies for cervical cancer in China. Highlights in Science, Engineering and Technology, 2, 24–29.
Zhou, L., Gu, B., Wang, J., Liu, G., & Zhang, X. (2022). Human papillomavirus vaccination at the national and provincial levels in China: A cost-effectiveness analysis using the PRIME model. BMC Public Health, 22(1), 777.
 write
write