1-hour AEAPTS Treatment
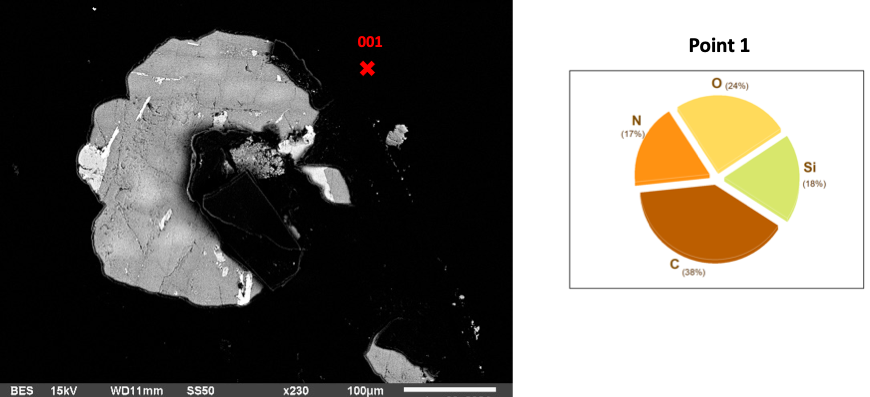
Fig 1: Basalt in AEAPTS for 1 hour
The one-hour AEAPTS treatment of basalt is crucial in material science, mainly surface engineering. AEAPTS, a silane coupling agent, creates a bonding bridge between materials [1]. An Energy Dispersive Spectroscopy (EDS) examination of the basalt surface after an hour of soaking and treatment with NaOH reveals alterations in its elemental composition with the highest silicon (Si) content (18%) on the basalt surface due to the short reaction period (Figure 1) and formation of Hydra Silica. Carbon makes up the highest chemical (38%), followed by oxygen at 24%, while Nitrogen is the lowest (17%).
3-hour AEAPTS Treatment
The 3-hour AEAPTS treatment basalt samples are substantial surface modifications that become increasingly apparent. The extended treatment duration primarily aims to improve bonding efficiency. Silicon content decreases from 18% to 14% alongside O, which drops from 24% to 20%, and C, which drops to 36%. N, however, increases to 29%. The Si coating at this point has reduced, and Silicon content has diffused into the basalt rock as the chemical reactions continue to form Hydra Silica (a combination of silicon and water molecules).
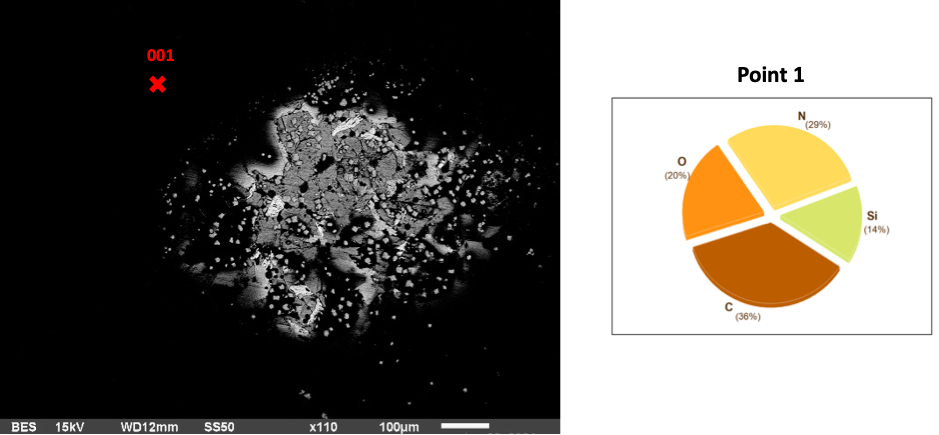
Fig 2: Basalt in AEAPTS for 3 hours
5-hour AEAPTS Treatment
When basalt samples are exposed to AEAPTS for 5 hours and treated with NaOH, there is a transformation in their surface composition and morphology. The continued exposure enables a denser and more uniform coverage of AEAPTS molecules on the basalt. EDS analysis at this stage shows a substantial decrease in silicon (Si) content on the basalt surface, which is lower than the hour and five-hour time frame. At this point, the surface of basalt rock has a coating comprising approximately 31% C, 28% O, 21% N and 13% Si. Oxygen is at its highest levels, while Si is at its lowest with the least surface coating.
This configuration change is indicative of an effective salinization method. The basalt’s chemical resistance is heightened, making it stronger and more capable of enduring harsh chemical situations [3]. It is primarily helpful for industrial usage where the material might be exposed to corrosive substances.
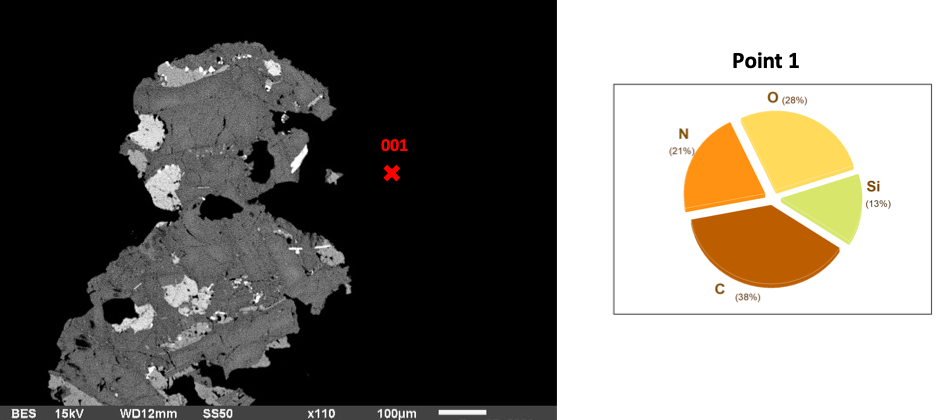
Fig 3: Basalt in AEAPTS for 5 hours
Reasons for Soaking Time
The research investigated the impacts of silane at different soaking times and subsequent NaOH treatment on the Si content on basalt surfaces. The outcomes showed that the Si content decreased with an increase in soaking time. The trend can be attributed to the reaction kinetics and the nature of the silane treatment process [4].
During the initial phase of treatment (1 hour), silanol (Si-OH) groups are gradually attached to the surface’s OH groups. The process could be faster and saturate all available OH groups on the fiber surface, causing higher Si content. As the soaking time is prolonged (three and five hours), most of the available OH sites on the fiber surface have already reacted with the silane molecules during the initial hours of treatment. Thus, fewer OH groups are available for further silane attachment, reducing Si content on the surface and forming Hydra Silica (Hydrated Silicon). Silicon component is thus highest with 1 hour of soaking and decreases gradually through the third hour, being lowest after 5 hours of soaking, with C depicting a similar trend. Oxygen increases after three hours and decreases after 5 hours of soaking, while N increases after 3 hrs and increases after 5 hours of soaking. The reaction reaches a saturation point where extra soaking time does not substantially increase the Si content.
References
Zhao, C., Li, L., Li, X., Chen, W., & Li, Y. (2023). Multi-scale analysis of the synergistic strengthening effect of silane coupling agent on PVA and cement interface. Frontiers in Materials, 10. https://doi.org/10.3389/fmats.2023.1276785
Narayanan, T. S. N. S., Park, I.-S., & Lee, M.-H. (2015). Silane Coating – an overview ScienceDirect Topics.
Ponzi, G. G. D., Santos, V. H. J. M. dos, Martel, R. B., Pontin, D., Stepanha, A. S. de G. e, Schütz, M. K., Menezes, S. C., Einloft, S. M. O., & Vecchia, F. D. (2021). Basalt powder as a supplementary cementitious material in cement paste for CCS wells: chemical and mechanical resistance of cement formulations for CO2 geological storage sites. International Journal of Greenhouse Gas Control, 109, 103337.
Najeeb, M. I., Sultan, M. T. H., Andou, Y., Shah, A. U. M., Eksiler, K., Jawaid, M., & Ariffin, A. H. (2020). Characterization of silane-treated Malaysian Yankee Pineapple AC6 leaf fiber (PALF) towards industrial applications. Journal of Materials Research and Technology, 9(3), 3128–3139. https://doi.org/10.1016/j.jmrt.2020.01.058
 write
write