Osmosis is one of the physiological processes that affect the movement of water molecules in and out of the cells. Osmosis is the movement of water molecules from a region of high water molecule concentration to an area of low molecule concentrations across a semi-permeable membrane (Li et al.). The osmosis process occurs in plant and animal cells because they have cells with different solute concentrations and membranes covering their cell contents. However, plant and animal cells respond differently when exposed to different osmotic environments. The plant cell has an extra membrane and cellulose cell wall that does not exist in the animal cell (Paulraj et al.). The additional compartment in the plant protects the cell’s inner membrane from bursting (Paulraj et al.). Therefore, there is a need to examine the behavior of cells when exposed to different environments using scientific experiments. The paper outlines a lab report that examines the behavior of cells when exposed to other osmotic conditions. The article begins by showing the methods used to experiment. Besides, the paper outlines the results and explains the meaning of the results. Finally, the paper compares the hypothesis, the results and the class results.
Method
Scientific evidence is gathered using appropriate research methods and design. The data of the study was collected using the experimental design method. The experimental setup is where the actual experiment is conducted, and results are tabulated based on practical observations. An investigation was carried out using a microscope and Elodea leaves. Besides, a control experiment was also set aside to compare the study findings. The experiment exposed the Elodea to hypotonic, hypertonic and isotonic solutions. Again, there was a hypothesis statement to support the study. The study hypothesises that cells behave differently when exposed to different osmosis environments.
Procedure
The osmotic environments are isotonic, hypotonic and hypertonic. The cells have diverse behaviors when exposed to these environments. Therefore, for this experiment, live cells are required. This would assist in displaying the actual behavior of the cells if exposed to these environments. The experiment uses the Elodea leaves to present the live cells. The steps of the investigation are outlined as follows.
- Make three wet slides and observe under a microscope. On the first slide, place an Elodea leaf and add 2 to 3 drops of tap water. On the second slide, put the Elodea leaf and add 2 to 3 drops of 20% sodium chloride (NaCl). Similarly, on the third slide, add 1% sodium chloride.
- Use a microscope to observe the two slides, paying attention to the cell wall and chloroplasts.
- Sketch the observation using the resolution of time X100.
Results
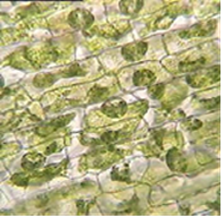
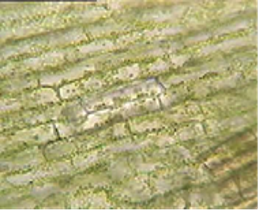
The results from the experiment are recorded in the table below.
| Distilled Water | 10% Sodium Chloride | 0.9% Sodium Chloride | |
| Cell Appearance | The chloroplasts were evenly distributed. | The chloroplast moved to the center of the cell | The chloroplast was evenly distributed. |
| Image Appearance |  |
 |
 |
Meaning of the result
Hypotonic Solution
The result from the table needs scientific interpretations. The first interpretation is on the elodea leaf placed in distilled water. The filtered water has a lower solute concentration as compared to the contents of the elodea leaf cells. In such a case, the distilled water is hypotonic (Fuentes and Entezari). Therefore, the water molecules move from a region of high concentration to an area of low concentration. In essence, the osmosis process is taking place. Osmosis involves the movement of water molecules from a region of high water molecule concentration to an area of low water molecule concentration across a membrane. The water moves into the cell across the semi-permeable membrane. The cells are filled, and the chloroplasts are not moved, but water pushes them towards the cell walls. The turgidity of the cell is developed because of the increase in the water content. However, the cells do not burst because of the cellulose cell wall. The cellulose cell wall is the non-living, fully permeable covering of the plant cell. The cell membrane of the plant cell rests on the walls of the cellulose; hence, it cannot burst.
Hypertonic Solution
Moreover, the cell, treated with 10% sodium chloride, had its chloroplasts shrinking towards the center of the cell. The 10% sodium chloride contents have higher solute concentrations than the cell’s solute contents (Rodrigues et al.). A normal elodea cell has a concentration of 1% sodium chloride. Therefore, it is essential to note that the elodea cell has a higher water concentration than the environment. Therefore, this solution is hypertonic. A hypertonic solution is one in which the solvent concentration of the domain is higher than the solute or solvent concentration of the cell. The process of osmosis in the cell would be reversed. The cell would lose its solvent to the external solution that contains more solute. Therefore, the cell becomes flaccid at the end. That makes the chloroplasts move to the center of the cell. The cell wall remains, covering the inner membrane that shrinks towards the center. The process of losing the solvent to the external environment results from osmosis.
Isotonic Solution
The last interpretation is on the normal cell. The regular elodea leaf shows an even distribution of the chloroplast throughout the cell. Besides, the chloroplasts do not appear puffy. In this case, the solute in the external environment and the solvent in the cell are at the same level. The cell and the environment are all at 1%. The solution in which the environment and the cell contents are at the same level is known as isotonic (Gassama and Özgür Öteyaka, 2019). The principle of osmosis operates at this level because no solution can attract each other. This means that water would not move out of the cell or enter the cell because of the equilibrium between the keys.
Hypothesis and the Results
My hypothesis was the cell environment influences the cell’s activity—the cell’s behaviour changes with the change in the background. The environment plays a critical role in enhancing cell physiology. The results of the experiment prove the hypothesis as accurate. The hypotonic solution influences the cell to absorb more solvent, making it puffy to the extent it can burst. The hypertonic solution makes the cell lose the solvent, making it flaccid. Finally, the isotonic solution does not influence the cell contents because of the existing equilibrium.
The Results in Comparison to the Class Results
The class data is similar to the results found from the experiment. The experiment indicates that the solutions are classified into three hy: photonic, hypertonic and isotonic. The results from the class show that hypotonic solutions make the cell turgid. However, the plant cell cannot break because of the cell wall that protects the inner membrane. The hypertonic solution makes the cell flaccid because it pulls the contents of the cell using the principles of osmosis. Besides, the control experiment indicates that the cell retained its contents. The cell environment and the contents were similar.
The Gaps for Further Research
The results show that osmosis is an essential process that makes the cell to grasp water and mineral salts. The cell, especially the plant cell, uses the root hair cell to take in water through the osmosis process. The contents of the root hair cell have low water molecule concentration. Therefore, it attracts the water from the region of high water concentration to an area of low water concentration across a semi-permeable membrane. However, an experiment that shows how the animal cell behaves in different osmotic environments has yet to be done. This indicates a gap in finding how the animal cell would behave.
Conclusion
The paper presents a lab report from an experiment. The primary aim of the experiment was to establish the behavior of cells in different osmotic environments. The third paper experiments with Elodea leaves using the hypotonic, hypertonic and isotonic solutions. The results indicate that movement of molecules exists in both the hypotonic by getting into and out of the cell in hypertonic. The paper concludes that all these occur as a result of osmosis. The report further recommends that there is a need to experiment to show how the animal cell behaves in all three environments.
Work Cited
Fuentes, Ana-Lucía, and Maria Entezari. “Water in your neighbourhood: a model for implementing a semester-long course-based undergraduate research project in introductory biology.” Education Inquiry 11.3 (2020): 211-275. https://doi.org/10.1080/20004508.2020.1716542
Gassama, Bassady, and Mustafa Özgür Öteyaka. “Influence of cryogenic treatment on the corrosion of AZ91 and AM60 magnesium alloys in an isotonic solution.” Materials Testing 61.11 (2019): 1039-1044. https://doi.org/10.3139/120.111420
Li, Na, et al. “Stoichiometric effect on the structural transformation and spatial variation of polyamide reverse osmosis membranes: A molecular dynamics study.” Journal of Membrane Science 686 (2023): 121980. https://doi.org/10.1016/j.memsci.2023.121980
Paulraj, Thomas, et al. “Primary cell wall inspired micro containers as a step towards a synthetic plant cell.” Nature Communications 11.1 (2020): 958. https://doi.org/10.1038/s41467-020-14718-x
Rodrigues, F. J., et al. “Encapsulated probiotic cells: Relevant techniques, natural sources as encapsulating materials and food applications–A narrative review.” Food Research International 137 (2020): 109682. https://doi.org/10.1016/j.foodres.2020.109682
 write
write