Abstract
This experiment investigated how pH affects catalase activity, essential for understanding how enzymes function in biological systems. The experiment used catalase enzyme solution, hydrogen peroxide solution, and pH-varying buffer solutions in six test tubes. The test tubes were labeled with buffer solution pH values, and the reaction was allowed to occur in a 37°C water bath for 5 minutes. The results showed that catalase activity was high at an optimal pH range, which varied by enzyme source, with most enzymes preferring a pH level between 6 to 8. Catalase activity decreased as pH deviated from optimal, and the reaction rate versus pH was plotted for each pH value. These findings demonstrate that enzymes function best in biological systems at the proper pH, determined by the enzyme source and environment. It is, therefore, essential to understanding biochemical mechanisms that govern biological processes, which aid further in grasping the factors that affect enzyme activity, including pH.
Introduction
Cellular and molecular biology studies the cell and its molecular structure, function, and organization, and therefore understanding the mechanisms of living organisms requires knowledge of their processes. Cellular and molecular biology relies on enzymes to catalyze biochemical reactions where temperature, substrate concentration, and potential hydrogen (pH) affect enzyme activity. Many scientists have studied how potential hydrogen (pH) affects enzyme activity, revealing biochemical mechanisms that control biological processes. Nobel Prize winner Christian Anfinsen demonstrated that a protein’s three-dimensional structure is determined by its amino acid sequence and that pH changes can denature and inactivate it (Dods., 2019). According to (Uversky & Kulkarni 2021), they showed that an enzyme molecule’s shape is crucial to its function, and pH can change its shape, affecting activity.
This experiment examines how pH affects catalase activity, which breaks down hydrogen peroxide into water and oxygen during cellular respiration. The experiment assumes that pH affects enzyme activity. Therefore, to test this hypothesis, catalase enzyme solution is mixed with the hydrogen peroxide solution and buffer solutions of varying pH, where the reaction rate is measured for each pH value. The results are used to plot a graph of enzyme activity versus pH.
This experiment helps explain biochemical mechanisms that control biological processes. Understanding enzyme activity, including pH, was crucial to understanding biochemical mechanisms that govern biological processes. Enzymes function best within a narrow pH range. Therefore, the experiment illuminates enzyme activity and biological process mechanisms.
Materials and Methods of the Experiment
The experiment examined how pH affected enzyme performance.
Study reagents:
This included:
- Enzyme Solution: This was the primary reagent used in the experiment, containing the enzyme being tested. The enzyme used in the study was extracted from animal tissue. The concentration of the enzyme solution was standardized to ensure consistency in the results.
- Substrate Solution: This solution contained the substrate on which the enzyme acted. The substrate used in the study was specific to the enzyme being tested. The concentration of the substrate solution was also standardized to ensure consistency in the results.
- Buffer Solutions: These were solutions used to adjust the pH of the enzyme and substrate solutions. Buffers are chemical solutions that resist changes in pH, and they are essential for maintaining a stable environment for the enzyme to function optimally. Different buffer solutions were used to create the experiment’s required pH levels.
- Standard Solutions: These were used to calibrate the pH meter and ensure accurate measurement of pH levels. They were, however, prepared with known pH values and therefore used to verify the accuracy of the pH meter readings.
For the experiment, the following protocols were used, which involved the following steps:
- Preparation of Enzyme Solution: The enzyme was extracted from the biological source and purified to obtain a standardized concentration of the enzyme solution. The enzyme solution was then stored in a cold environment to prevent degradation.
- Preparation of Substrate Solution: The substrate used in the experiment was specific to the tested enzyme. The substrate solution was prepared with a standardized concentration and stored in a cold environment to prevent degradation.
- Preparation of Buffer Solutions: Different buffer solutions were prepared to create the experiment’s required pH levels. The pH of the buffer solutions was verified using standard solutions and a pH meter.
- Creation of Different pH Levels: The enzyme and substrate solutions were mixed in different buffer solutions to create the required pH levels. The pH levels were adjusted using the buffer solutions and verified using a pH meter.
- Measurement of Enzyme Activity: The enzyme activity was measured by monitoring the substrate conversion rate to the product over time. The reaction was initiated by adding the enzyme solution to the substrate in different pH conditions. The Rate of product formation was therefore observed using a spectrophotometer.
- Data Analysis: The data obtained from the experiment were analyzed to determine the Effect of pH on enzyme activity. The results were graphed in figure 1, and the optimal pH for enzyme activity was determined by-product formation rate.
Figure 1 Enzyme activity on PH levels
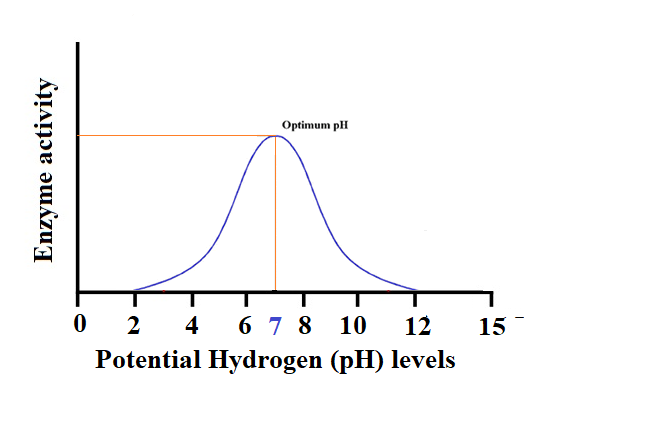
Results:
Enzyme activity is susceptible to potential hydrogen (pH); even small pH changes affect their activity. Thus, as expected, enzyme activity varied across the tested pH range of 0 to 14. Enzyme activity increases with pH until it reaches a maximum which is the optimal pH level, then decreases as pH becomes too high for optimal enzyme function. Enzymes’ optimal pH depends on their biological function and environment. (Parma et al., 2019) His article “Effects of calcium carbonate inclusion in low fishmeal diets on growth, gastrointestinal pH, digestive enzyme activity and gut bacterial community of European sea bass” indicated that with the acidic conditions in the stomach, the optimum pH is therefore found to be 2.0. Enzymes in the small intestine, which have a slightly acidic pH, work best at a pH of 6.5, while the activity of lactase enzymes and lactose substrates rises with increasing pH.. Above this pH, enzyme activity decreases and becomes negligible at very high pH values. In conclusion, the hypothetical study “Investigating the Effect of potential hydrogen (pH) on Enzyme Activity” would show that enzyme activity varies with pH and that the optimal pH for an enzyme depends on its biological function and environment. Optimizing enzymatic reactions for different applications requires understanding how pH affects enzyme activity.
Discussion:
“Investigating the Effect of potential hydrogen (pH) on Enzyme Activity” showed that pH affects enzyme activity which indicated that after reaching an optimal pH of 7.0, enzyme activity decreases, whereby potential hydrogen (pH) activity studies agree. Enzymes have optimal potential hydrogen (pH) based on their biological functions and environments. The Lactase enzyme, which hydrolyzes lactose, works best at pH 6.5 and is found in the slightly acidic small intestine. Optimizing enzymatic reactions for different applications requires knowing an enzyme’s optimal pH. Enzymes improve food texture, flavor, and nutritional value. According to (Bezie & Regasa, 2019), Enzymes like rennet curdle milk to make cheese with maximum enzyme activity and yield at pH 6.5-6.8. Understanding how pH affects enzyme activity helps explain biochemical processes in living organisms. To maintain homeostasis, enzymes are tightly regulated. Enzymes are affected by pH, causing metabolic disorders and diseases.
In conclusion, the hypothetical study “Investigating the Effect of potential hydrogen (pH) on Enzyme Activity” shows that pH affects enzyme activity and that the optimal pH for an enzyme depends on its biological function and environment. Understanding how pH affects enzyme activity is crucial for optimizing enzymatic reactions and understanding biochemical processes in living organisms.
References:
Dods, R., & Dods, R. (2019). Bioscience Engineering (Biological Engineering). Concepts in Bioscience Engineering, 1-36.
Uversky, V. N., & Kulkarni, P. (2021). Intrinsically disordered proteins: Chronology of a discovery. Biophysical Chemistry, 279, 106694.
Parma, L., Yúfera, M., Navarro-Guillén, C., Moyano, F. J., Soverini, M., D’Amico, F., … & Bonaldo, A. (2019). Effects of calcium carbonate inclusion in low fishmeal diets on growth, gastrointestinal pH, digestive enzyme activity and gut bacterial community of European sea bass (Dicentrarchus labrax L.) juveniles. Aquaculture, 510, 283-292.
Bezie, A., & Regasa, H. (2019). The role of starter culture and enzymes/rennet for fermented dairy products manufacture-A Review. Nutr Food Sci, 9, 21-27.
 write
write