A Pourbaix diagram, which is also known as a potential-pH diagram, is a graphical representation of the thermodynamic stability of various chemical species as a function of the pH and the electrode potential (E) under particular conditions, such as temperature and pressure. A Pourbaix diagram can be found in the literature under the name potential-pH diagram (Gerken et al., 2011). While developing a Pourbaix diagram for Cobalt, it is necessary to take into account the key chemical processes as well as the thermodynamic data associated with them. The following equations represent some of the responses that it is necessary for us to take into consideration:
Cobalt oxidation: Co → Co2+ + 2e- E° = -0.28 V
Cobalt hydrolysis: Co2+ + 2H2O ↔ Co(OH)2 + 2H+ K = 2.0 × 10^-15
Cobalt oxide formation: Co2+ + 2H2O + 1/2O2 ↔ Co(OH)2(s) K = 1.6 × 10^-42
Cobalt hydroxide formation: Co2+ + 2OH- ↔ Co(OH)2 K = 4.0 × 10^-16
Cobalt oxide reduction: Co3O4 + 8H+ + 6e- ↔ 3Co2+ + 4H2O E° = 0.54 V
Using these equations and data, we can create the following Pourbaix diagram for Cobalt:
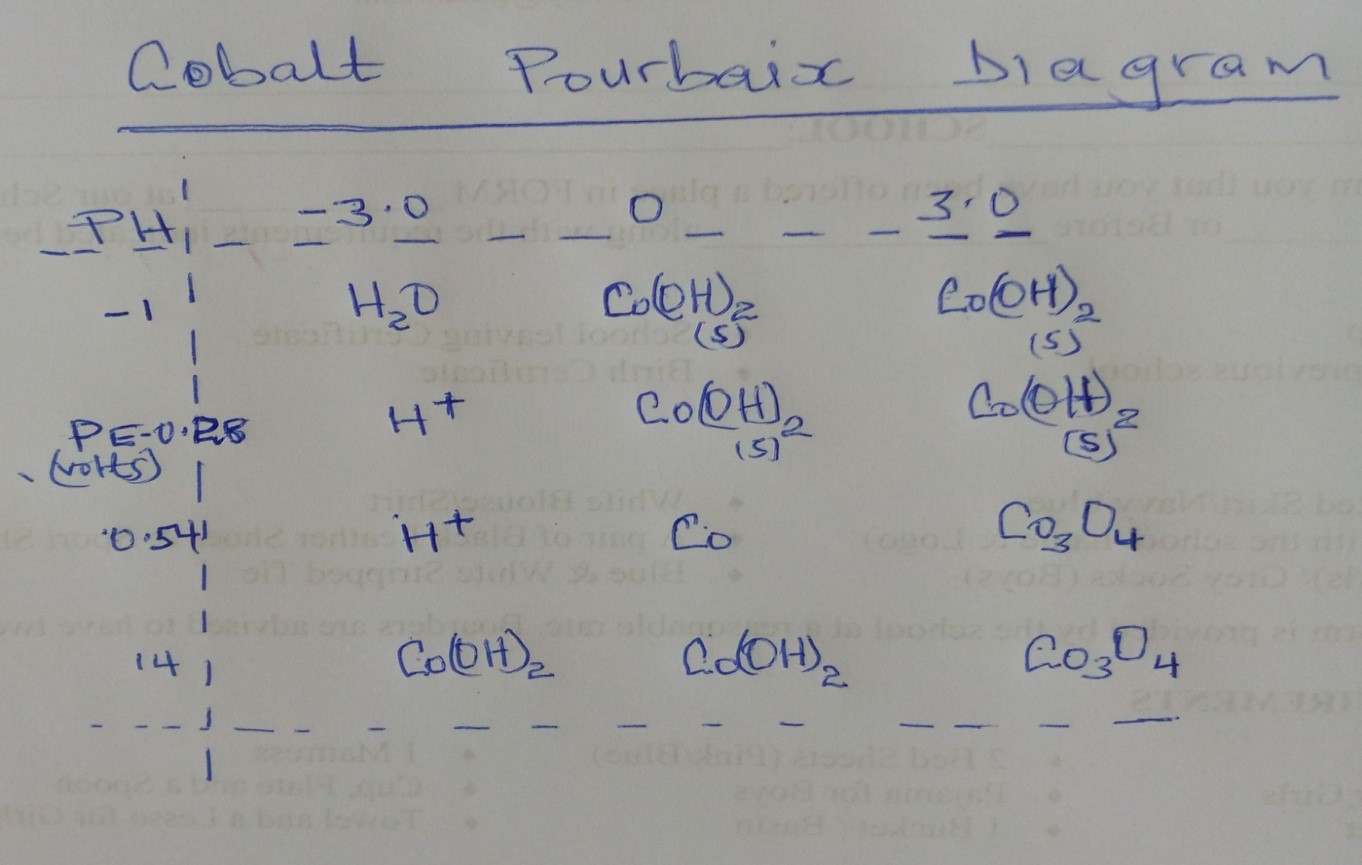
In the above diagram, the electrode potential (PE) is shown on the horizontal axis, while the pH is located on the vertical axis. The various sections of the picture each relate to a distinct stable form of Cobalt that may be formed under a variety of circumstances. The lines with dots reflect the equilibrium between multiple species, whilst the lines with solids represent the stability limits for each species individually.
In solutions with low pH values and negative electrode potentials, Cobalt may be found in the form of the ion Co2+. When the pH is raised, the hydrolysis of Co2+ to generate Co(OH)2 becomes more favourable, and we witness a region where the stable form of Cobalt is Co(OH)2(s). The reduction of cobalt trioxide (Co3O4) gets increasingly favourable when the electrode potential is increased, and we see a region in which cobalt trioxide is the stable form of the element. At higher pH levels, cobalt hydroxides become more stable, including Co(OH)2 and Co(OH)3.
The Pourbaix diagram may also be used to predict the circumstances that will be present throughout the course of specific chemical processes. If we wanted to electro-deposit Cobalt from a solution that contained cobalt ions (Co2+), for instance, we would have to work at a potential that was lower than -0.28 volts and at a pH that was lower than the line that divided cobalt ions from cobalt hydroxyl radicals (Co(OH)2(s).
It is also possible to draw a graphical representation of the Pourbaix diagram as shown below:
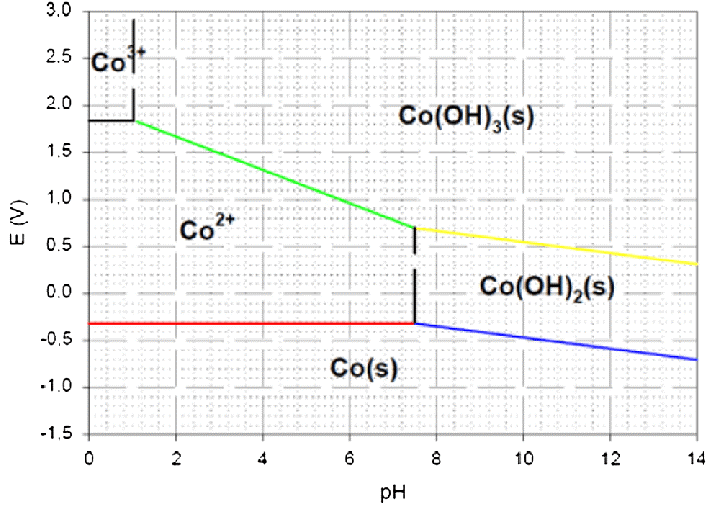
The stability of the metal is shown in the graph as a function of the pH of the solution and the electrode potential (E). The electrode potential is measured in volts (V) in relation to the standard hydrogen electrode, which may be found along the y-axis. The solution’s pH is shown on the x-axis of the graph.
The different phases and species of Cobalt that are stable under particular circumstances are shown by the different zones of the figure. The Pourbaix diagram begins with the cobalt-water system and depicts the creation of several cobalt species as well as the stability of those species as a function of pH and potential.
Cobalt occurs as the soluble Co2+ ion at pH levels lower than 4.5. When the pH of the solution is lower, and the potential is higher, the amount of stable Co2+ rises. The Co2+ ion is unstable at greater negative potentials, and it may be converted to metallic Cobalt in certain circumstances.
Cobalt may be found in the form of cobalt hydroxide, or Co(OH2), with pH values between 4.5 and 9. As the pH of the solution is raised and the potential is lowered, the amount of stable Co(OH)2 rises. When exposed to potentials that are more positive, cobalt hydroxide is unstable and may be oxidized to cobalt oxide (Co3O4).
Above pH 9, Cobalt exists as cobalt oxide, Co3O4. The stability of Co3O4 increases with increasing pH and potential. At more negative potentials, Co3O4 can be reduced to Co(OH)2.
Above pH 13, Cobalt exists as the soluble Co(OH)4 2- ion. The stability of Co(OH)4 2- increases with increasing pH and potential. At more negative potentials, the Co(OH)4 2- ion is unstable and can be reduced to Co(OH)2.
The Pourbaix diagram is only able to provide predictions about the system’s thermodynamic stability; it does not take into account kinetics or any other aspects that can have an effect on how the system actually behaves. The Pourbaix diagram for Cobalt, in general, offers a wealth of information on the stability of cobalt species under a variety of circumstances and may be used to make accurate predictions regarding the corrosion behaviour of Cobalt in aqueous environments (Zhou et al.,2022).
References
Gerken, J. B., McAlpin, J. G., Chen, J. Y., Rigsby, M. L., Casey, W. H., Britt, R. D., & Stahl, S. S. (2011). Electrochemical water oxidation with cobalt-based electrocatalysts from pH 0–14: the thermodynamic basis for catalyst structure, stability, and activity. Journal of the American Chemical Society, 133(36), 14431-14442. https://pubs.acs.org/doi/abs/10.1021/ja205647m
Powell, D., Cortez, J., & Mellon, E. K. (1987). A laboratory exercise introducing students to the Pourbaix for Cobalt. Journal of Chemical Education, 64(2), 165. https://pubs.acs.org/doi/pdf/10.1021/ed064p165
Zhou, L., Li, H., Lai, Y., Richter, M., Kan, K., Haber, J. A., … & Gregoire, J. M. (2022). Stability and activity of cobalt antimonate for oxygen reduction in strong acid. ACS Energy Letters, 7(3), 993-1000. https://pubs.acs.org/doi/abs/10.1021/acsenergylett.1c02673
 write
write