Introduction
Grignard reaction is vital to organic C-C bond formation. The organohalide reacts with Mg metal to form the Grignard reagent, alkyl magnesium halide (Pietrasiak & Lee, 2022). Figure 1 shows the halide reduced and magnesium oxidized in the redox reaction under ether.
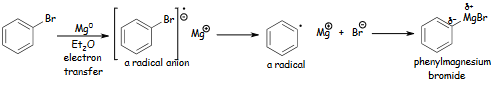
Figure 1: A depiction of the formation of a Grignard reagent
An electron is transported from magnesium metal to bromobenzene, generating a radical anion intermediate containing an unpaired electron and anion. The C radical combines with unstable Mg+1 to generate the Grignard reagent. Due to radical coupling, the reagent may produce side products. Since C is more electronegative than Mg, the C-Mg bond is strongly polarized and partially negative. This renders the reagent a carbanion, nucleophile, and strong base. The feature also requires that weak acids like water and alcohol be avoided during reagent synthesis to avoid proton transfer side products. The Grignard reagents react with diethyl ether or tetrahydrofuran, and the lone pair of electrons on oxygen stabilizes the partial positive charge on Mg to generate the Grignard reagent (Alabugin et al., 2021). The reagent can nucleophilically add C-C bonds to several carbonyl-containing chemical entities. Polarity at the carbonyl carbon causes electrophilicity. Acidified Grignard reagents create secondary and tertiary alcohols from aldehydes, ketones, and esters. Figure 2 shows that the reagent combines with CO2 under acidic circumstances to create carboxylic acids, while water yields an alkane.
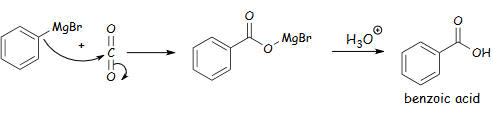
Figure 2: A depiction of a Grignard reagent reacting with CO2 to form a carboxylic
The reaction in Figure 2 can be conducted by bubbling CO2 gas into a solution of Grignard reagent or conveniently pouring the Grignard reagent onto dry ice. The procedure yields a magnesium carboxylate intermediate, then treated with strong acids like HCl when reworking. The acid also decomposes any residual magnesium metal, so the magnesium salt formed dissolves in the aqueous layer for easier purification. In this reaction, biphenyl is the byproduct of the reagent’s heat or light-catalyzed coupling reactions with the unreacted bromobenzene, as shown in Figure 3.
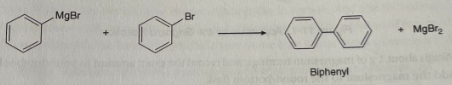
Figure 3: A depiction of the formation of biphenyl
Results and Discussion
The jar without the product was 80.3 g
The jar with the product was 82.0 g
The mass of the product was (82.0 – 80.3) = 1.7 g
Computing for the theoretical mass of benzoic acid
Bromobenzenedensity = 1.5 g/cm3
Given 5mL of bromobenzene was used, the number of masses of bromobenzene reacting would = Volume x Density = 5mL × 1.5 g/cm3 = 7.5g
Bromobenzenedensity = 1.5 g/cm3
Mole of bromobenzene = 7.5g/157.01 g/mol = 0.04777mol
Given that bromobenzene and magnesium react on a 1:1 mole ratio, the number of moles of Mg = 0.04904 mol
The phenylmagnesium bromide formed will also have 0.04777 mol
Therefore, its mass = 0.04777 × 181.31g/mol = 8.6608g
The phenylmagnesium bromide also combines with carbon (IV) oxide on a 1:1 mole ratio hence the number of moles of CO2 required and the benzoic acid expected to be formed = 0.04777 mol
The theoretical mass of the benzoic acid = 0.04777 mol × 122.12g/mol = 5.8334g
%yield = (yield from experiment/ yield from theory) × 100% = (1.7g/5.8334g) ×100% = 29.14259199% ≈ 29.14%
Anhydrous ether is an aprotic solvent that reacts with the Grignard reagent to generate a stable and soluble complex with a remarkable ability to react and prevent alkanes, alkenes, and alkynes. Anhydrous CaCl2 dehydrates the reaction system to remove moisture that preferentially combines with the Grignard reagent to create byproducts. 6M HCl transforms the MgBr2 salt to benzoic acid and dissolves excess Mg metal to generate a solution of mineral salts, HCl, and an ether layer with neutral organic compounds.
Conclusion
In this experiment, we synthesized benzoic acid with a mass of 1.7g. The product deviated from the theoretical yield of 5.8334g and had a percentage yield of 29.14%. Nonetheless, we achieved our experimental aim of preparing benzoic acid from our synthesized Grignard reagent.
References
Alabugin, I. V., Kuhn, L., Medvedev, M. G., Krivoshchapov, N. V., Vil, V. A., Yaremenko, I. A., Mehaffy, P., Yarie, M., Terent’ev, A. O., & Zolfigol, M. A. (2021). Stereoelectronic power of oxygen in control of chemical reactivity: The anomeric effect is not alone. Chemical Society Reviews, 50(18), 10253–10345.
Pietrasiak, E., & Lee, E. (2022). Grignard reagent formation via C–F bond activation: A centenary perspective. Chemical Communications, 58(17), 2799–2813.
 write
write