Advancement in organic compounds has compelled modern-day scholars to focus more on azo and diazo compounds. According to Mohr (2013), azo and diazo are two terminologies referring to valuable compounds in organic chemistry. Whereas azo is the naming word for compounds with the functional group (N=N), its counterpart refers to the active group of whole molecules that have an a-N2 group. The diazo group of organic compounds exists at the molecular terminal. Despite being a unique category of organic compound, each group has distinct structural attributes that differentiate it from the others (Goldberg, 2015). Likewise, each category of these organic compounds has a different reaction habit when exposed to other chemical elements.
Structural Components of Azo and Diazo Compounds
Azo is a particular functional group (N=N) attributing a two-phased bond between two nitrogen atoms. In this regard, azo compounds are a group of organic molecules containing a unique active group in their molecular structure (Farr et al., 2001). The standard structure of an azo compound is R-N=N-R, where the two Rs represent organic groups attaching to nitrogen atoms. Likewise, the original attributes of the two Rs drive the azo compound’s properties, such as solubility, color, and stability. The azo compound’s color emerges from its structure’s fused pair of bonds. Combining the N=N pair of bonds alongside the carbon-carbon double aromatic rings or bonds causes the absorption of specific light wavelengths, influencing color display (Mohr, 2013). More so, azo dyes can help generate a vast color spectrum, presenting azo dyes as unique and versatile.
Most azo dyes go through aromatic primary amine before intergrading them with one or more electrons with abundant nucleophiles like hydroxyl and amino. Other approaches to synthesizing the azo dyes include lowering nitroso compounds using AILH4, reducing nitroaromatic derivatives in an alkaline medium, and using lead tetraacetate or permanganate potassium to oxidate the primary amines. Likewise, condensation of the main amines using nitroso derivatives can also perform this role (Stehn, 2004). Besides, the azo compounds can also interlink to form benzene rings, aromatic heterocycles, naphthalenes, or enolizable aliphatic groups, which are critical in coloring dyes and creating different levels of shades. The chemical representation of an azo dye manifests through a backbone, chromophoric, auxochrome and solubilizing groups as seen in diagram 1.
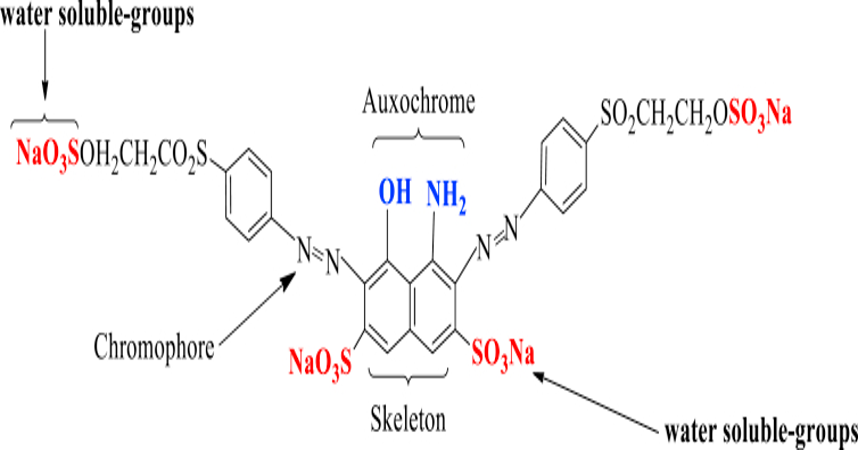
Diagram 1: The structure of an azo-reactive dye
Most of the azo dyes, including the one depicted above, show a chemical group that can interact with textile substrates to form covalent bonds. The action needed for the bonds’ rupture correlates with the energy that facilitates degradation for self-support.
Classifying Azo Dyes in Group Number
Accordingly, chemists classify azo dyes based on the number of azo linkages (uniform molecules of the dyes like diazo, monoazo, polyazo, trisazo, and azoic. The distribution of azo dyes occurs in numbers from 11,000 to 39999, in line with the chemical structures that influence the color index system, as seen in Table 1. The society of colorists and dyes developed the color index number, which facilitates dye classification.
| Chemical class | Color Index Number |
| Monozo
Disazo Trisazo Polyazo Azoic |
11,000-19999
20000-29999 30000-34999 35000-36999 37000-39999 |
Table 1: Classification of azo dyes in the CI system
Diazo compounds often have a unique diazo functional group (-N2), have significant reactivity patterns, and are a versatile median element in organic synthesis. This 19th-century discovery by Griess Peter has a nitrogen-nitrogen double bond, which creates considerable energy and influences a wide range of transformations (BGFA, 2009). As a result, most chemical innovators capitalize on diazo compounds’ unique reactivity to create advanced materials, reactions, and pharmaceuticals.
There are three methods of synthesizing diazo dyes: primary diazo, symmetric, and asymmetric syntheses. The primary diazo entails the coupling reaction of two molecules of diazoic acid under uniform coupling conditions (Mohr, 2013). The resulting dyes are derivatives of resorcinol and m-phenylenediamines with green, brownish, black, and matt blue appearance. Diagram 2 depicts a typical example of a brown dye essential in dying wool.
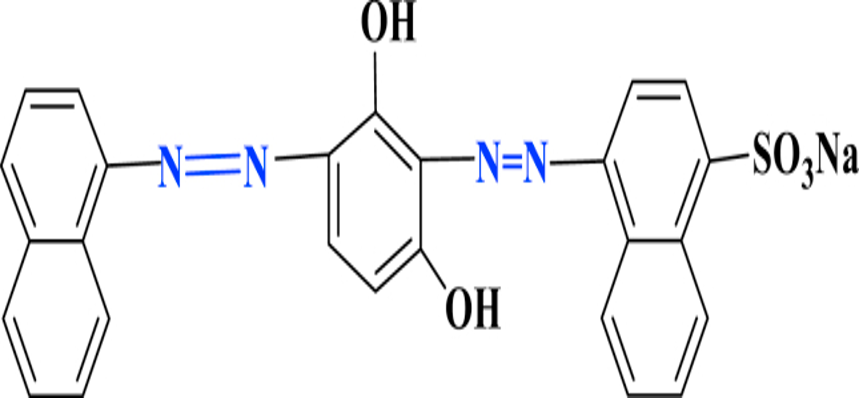
Diagram 2: A depiction of a brown dye structure
The symmetrical method entails the double diazotization and copulation of diamine with different or identical terms. Diagram 3 shows the structure of a blue dye with benzidine functions.
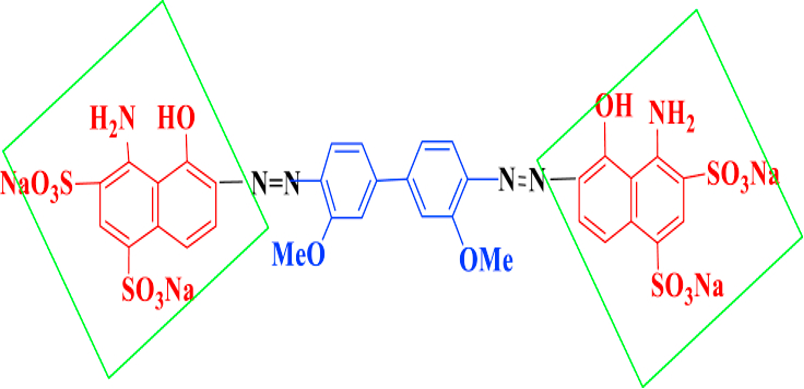
Figure 4: The blue direct dye structure
Finally, the asymmetrical method requires chemists to couple an amino azoic with a phenolic coupler for synthesis. For instance, the orange direct dye, whose structure is in Figure 5, is the product of the asymmetrical method.
Similarities and differences between Azo and Diazo Compounds
Some similarities between azo and diazo compounds are that they have an azo group, which manifests as N=N. Secondly, these compounds show significant reactivity due to a nitrogen-nitrogen double bond. More so, most groups of the azo and diazo compounds, especially the azo dyes, depict bright colors (Fahr et al., 2001). Both compounds help generate complex molecules through organic synthesis. Besides, azo and diazo compounds vary in definition, structure, stability, and applicability. Whereas azo compounds are organic molecules containing a unique N=N group with bright colors and reactivity attributes, diazo has an a-N2 group and depicts significant reactivity. Structural-wise, azo exists as R-N=N-R, with the two Rs representing organic groups that dwell on the nitrogen atom, while diazo’s structure comprises –N2 (a diazo functional group). In the same context, azo is more stable than diazo, with the latter depicting significant reactivity due to its diazo group (-N2). Concerning their applicability, azo compounds, especially the azo dyes, use their bright colors to impact the cosmetic and textile industries, while diazo drives organic synthesis (Stehn, 2004). Here, the diazo compound is essential for introducing functional groups and formulating complex molecules, spurring innovations in pharmaceuticals, reactions, and materials.
Uses of Azo and Diazo Compounds
Azo, an elevated subgroup of the azo compound, has changed many industries in recent decades. In particular, the azo dyes comprise a wide range of synthetic dyes, which dominate fields of plastics, pharmacy, living cell cancer, hypnotic medicine, and pharmacological procedures. These dyes are common in biological and complex technological applications like nonlinear optical systems, lasers, fuel cells, and thermal transfer printers. These dyes are also critical in making solar cells, metallichromic indicators, photodynamic therapy, textile dyeing cosmetics, food, leather, medicines, and other necessary fields (Goldberg, 2015). In the same context, azo dyes have many optical properties and significant physiochemical stability alongside many applications in nanotubes and liquid crystals.
Azo dye’s synthesis consists of a series of reactions in which a diazonium salt from aromatic amine interacts with a coupling agent, such as an aromatic compound with electron-donating groups to connect the azo (N=N) and orchestrate the colorful azo dye. Azo dyes’ ability to produce many colors, such as bright, vivid shades and subtle tones, makes them more advantageous (Mohr, 2013). Likewise, these compounds’ versatility makes them the best among many dyes in the clothing, plastics, leather, and food sectors. Besides dying, azo compounds are pivotal in producing pharmaceutical products. According to studies, these compounds are critical in synthesizing drugs and prodrug production and reinforcing bioavailability. Azo compounds’ unique attributes, such as photoisomerization, enable them to integrate with many functional elements like optical switches, organic semiconductors, and liquid crystals (BGFA, 2009). This phenomenon makes them valuable in the optoelectronic application. Also, these compounds’ light-induced structural transformative attributes make them suitable for nanotechnology and molecular machines. As a result, azo compounds are helpful in the innovation of light-responsive materials for the new versions of nanotech and drug delivery applications.
Diazo compounds such as diazomethane, a highly explosive and toxic yellow gas that exists as laboratory ether solution, are often helpful in converting carboxylic acids into their homologs or methyl esters. More significantly, the diazo transfer reaction is more practical, where the diazo group navigates toward the other molecule to regenerate diazo-related products and compounds. This critical organic synthesis process stimulates tailored attributes in various products (Fahr et al., 2001). More so, diazo compounds have changed organic processes and helped establish novel strategies and reactions. Their unique characteristics have helped them create reactive intermediaries and carbines, pivotal in formulating cyclopropane rings and carbon-hydrogen bonds. Likewise, these compounds’ reactions alongside the alkenes spearhead cyclopropanation reactions help generate essential components of complex molecular synthesis called the cyclopropane rings. These compounds’ distinct reactivity makes them suitable for metal-catalyzed conversions, facilitating cross-coupling reactions for carbon-nitrogen and carbon-carbon bond formation, essential in developing intricate molecules ( Mohr, 2013). Also, diazo compounds are helpful in synthesizing pharmaceutical and natural products, which pave the way for many groups of biologically active structures. These structures happen through the introduction of a selective diazo group.
Conclusion
Advancement in organic compounds has compelled modern-day scholars to focus on azo and diazo discourse. Massive production and application of azo dyes and the increasing demand for these components make the azo compounds and their discussion valuable in modern times. The pharmaceutical, food, textile, cosmetic, and leather sectors rely on more than 50% of the synthesized dyes. More so, the chemical reaction of azo coupling, which occurs between aromatic amines, naphthols, and amines, causes brightly-colored items called azo dyes. Besides, diazo is a distinct version of organic compounds that are different despite sharing some attributes with azo compounds. According to studies, diazo compounds have different chemical reactions, structures, and applications to azo compounds. Thus, exploring these compounds could provide valuable insights into how they could help impact our lives.
References
BGFA (2009). Aofarbmittel und deren Hautgangigkeit beim Menshen. Forschungsinstitut fur Arbeitsmedizin der Deutschen Gesetzlichen Unfallversicherung, 5 Fenruar. https://www.dguv.de/medien/ipa/publikationen/ipa-reporte/09-02-27_bgfa-report2_azofarbstoffe.pdf
Goldberg A. (2015). Zur titrimetrischen stickstoffbestimmung in Nitro-, Azo- Und Diazoverbindungen. Chemistry Europe: Wiley Online Library, pp.2546-2556.https://doi.org/10.1002/cber.188301602183
Fahr E., Keil K., Lind H. und Scheckenbah (2014). Untersuchungen an Diazoverbindungen. Zeitschrift für Naturforschung B, 2. Juni.https://doi.org/10.1515/znb-1965-0607
Fahr E., Doppert K. & Konigsdorfer K.(2001).Die umsetzung von Diazoverbindungen mit azoverbindungen-VI: Die umsetzung der silber- verbindungen von monoacyhydrazonen mit acylchloriden zu 1,3,4-oxidiazolinen BZW. Diacyl-hydrazonen. Institut fur organische Chemie der Universitat Wurzburg Germany.https://doi.org/10.1016/0040-4020(67)85092-0
Mohr E. (2013). Uber Diazo- nnd Azoverbindnngen der Pyrazolreihe. US-Archive, 8. June.https://ia800708.us.archive.org/view_archive.php?archive=/28/items/crossref-pre-1923-scholarly-works.
Stehn M. B. (2004). Synthese von Azo-bis(benzokronenethern) und Versuche zu deren Anwendung als molekulare Synthesemaschinen. Institut für Organische Chemie der Christian-Albrechts-Universitat zu Kiel agefertigt, 10, August. https://www.otto-diels-institut.de/herges/download/arbeiten/buchheim_stehn_diplomarbeit_2004.pdf
 write
write